RESEARCH/REVIEW ARTICLE
Aspartic acid racemization rate in narwhal (Monodon monoceros) eye lens nuclei estimated by counting of growth layers in tusks
Eva Garde,1,2 Mads Peter Heide-Jørgensen,2 Susanne Ditlevsen3 & Steen H. Hansen4
1 Center for GeoGenetics, Natural History Museum of Denmark, University of Copenhagen, Øster Voldgade 5–7, DK-1350 Copenhagen Ø, Denmark
2 Greenland Institute of Natural Resources, PO Box 570, DK-3900 Nuuk, Greenland
3 Department of Mathematical Sciences, Center of Applied Statistics, University of Copenhagen, Universitetsparken 5, DK-2100 Copenhagen, Denmark
4 Department of Pharmaceutics and Analytical Chemistry, Faculty of Pharmaceutical Sciences, University of Copenhagen, Universitetsparken 2, DK-2100 Copenhagen, Denmark
Abstract
Ages of marine mammals have traditionally been estimated by counting dentinal growth layers in teeth. However, this method is difficult to use on narwhals (Monodon monoceros) because of their special tooth structures. Alternative methods are therefore needed. The aspartic acid racemization (AAR) technique has been used in age estimation studies of cetaceans, including narwhals. The purpose of this study was to estimate a species-specific racemization rate for narwhals by regressing aspartic acid D/L ratios in eye lens nuclei against growth layer groups in tusks (n=9). Two racemization rates were estimated: one by linear regression (r2=0.98) based on the assumption that age was known without error, and one based on a bootstrap study, taking into account the uncertainty in the age estimation (r2 between 0.88 and 0.98). The two estimated 2kAsp values were identical up to two significant figures. The 2kAsp value from the bootstrap study was found to be 0.00229±0.000089 SE, which corresponds to a racemization rate of 0.00114−yr±0.000044 SE. The intercept of 0.0580±0.00185 SE corresponds to twice the (D/L)0 value, which is then 0.0290±0.00093 SE. We propose that this species-specific racemization rate and (D/L)0 value be used in future AAR age estimation studies of narwhals, but also recommend the collection of tusks and eyes of narwhals for further improving the (D/L)0 and 2kAsp estimates obtained in this study.
Keywords
Age estimation; Greenland; species-specific racemization rate; growth layers groups.
Correspondence
Eva Garde, Center for GeoGenetics, Natural History Museum of Denmark, University of Copenhagen, Øster Voldgade 5–7, DK-1350 Copenhagen Ø, Denmark. E-mail: egarde@snm.ku.dk
(Published: 21 November 2012)
Polar Research 2012. © 2012 E. Garde et al. This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial 3.0 Unported License (http://creativecommons.org/licenses/by-nc/3.0/), permitting all non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Citation: Polar Research 2012, 31, 15865, http://dx.doi.org/10.3402/polar.v31i0.15865
The tusk of the narwhal (Monodon monoceros) was previously thought to be the long horn of a mythical creature: the unicorn. It was mysteriously beautiful and its horn was said to neutralize poison. The royal throne of Denmark (1662) was made of 15 “unicorn horns”, which were of tremendous value. Back then, as today, narwhals were hunted for their valuable tusks. Today, only Inuit in Greenland and Canada are allowed to hunt narwhals, and the hunt is managed in both countries (NAMMCO 2009). Ongoing studies of migration patterns (Heide-Jørgensen et al. 2003), population sizes (Heide-Jørgensen et al. 2010; Richard et al. 2010) and life history (Garde et al. 2007) of narwhals contribute to understanding the underlying population dynamics of this exploited species (Reeves & Heide-Jørgensen 1994). Sustainable harvest levels are usually calculated from population dynamics models where survival and reproductive rates, based on reliable age distributions, are vital (Kingsley 1989). Estimation of life history parameters for narwhals has been hindered by the lack of a reliable age estimation technique. Without such a technique, it is difficult to parameterize rates of body growth, age at sexual maturity, longevity and survival rates of the whales.
Traditionally, ages of marine mammals have been estimated by counting dentinal growth layer groups (GLGs) in teeth, usually consisting of an opaque and a translucent layer deposited annually (Dietz et al. 1991; Heide-Jørgensen et al. 1994; Stewart et al. 2006; Lockyer et al. 2007). Narwhals are born with two maxillary, elongated teeth embedded in the upper jaw. The left tooth erupts through the lip in males, and as the whale grows older this becomes a long, spiralled tusk (Hay & Mansfield 1989). Normally, the right tooth remains inside the maxilla in males. In females, both teeth usually stay embedded in the maxillae. Anomalies such as females with tusks or males without a tusk or with two tusks are reported in the literature but occur infrequently in narwhal populations (Hay & Mansfield 1989). Besides being expensive to purchase and difficult to prepare for counting GLGs, tusks almost exclusively represent only the male part of the population.
Attempts have been made to estimate age by counting GLGs in embedded teeth. However, due to occlusion of the pulp cavity and thereby cessation of deposition of growth layers, only a minimum estimate of age can be obtained from these teeth for adults; reliable estimates can be obtained only for young individuals (Hay 1980). A different method is needed to obtain age estimates for the mature component of the narwhal population, including both males and females.
The aspartic acid racemization (AAR) technique has been used in several age estimation studies of cetaceans (Nerini 1983; George et al. 1999; Olsen & Sunde 2002). This technique relies on the fact that in metabolically inactive tissues, the l-form of aspartic acid converts to the D-form at a constant rate over time, a process known as racemization (Masters et al. 1977). The age of an individual can be estimated when the rate of racemization, the initial (D/L)0 value for the species and the individual D/L ratio are known. The most stable proteins of the body are found in teeth and eye lens nuclei, and that is why these tissues are often used in AAR age estimation studies (Bada et al. 1980). Bada et al. (1983) and Garde et al. (2007) used AAR to estimate ages of narwhals from teeth and eye lens nuclei. Bada et al. (1983) used the human racemization rate in teeth, whereas Garde et al. (2007) estimated a rate based on a combination of narwhal and fin whale (Balaenoptera physalus) data. The rate of racemization has been shown to differ among species, mainly driven by core body temperature (Garde 2011; Rosa et al. 2012). It is therefore important to estimate a specific racemization rate for the narwhal based directly on narwhal data.
The purpose of this study was to estimate a species-specific racemization rate for the narwhal by regression of GLGs in tusks against aspartic acid D/L ratios in eye lens nuclei. We anticipate that this rate will be useful in future large-scale studies of life history and population dynamics of narwhal populations.
Methods
Tusks and both matching eyes from 11 narwhals were purchased from the Inuit hunt in north-west and East Greenland in the period September 2006–November 2010 (Table 1). Eye samples from two whales (ID nos. 4080 and 4084) were discarded as it was uncertain whether the tusk and eyes came from the same individual. Eight of the remaining nine whales were males. We believe the ninth whale (ID no. 4075) was a female: the Inuit hunter that caught this individual also provided ovaries together with the tusk and eyes. Body lengths of the whales ranged from 306 to 491 cm (Table 1).
Table 1 Data on the 11 narwhals from North Greenland (NGl), West Greenland (WGl) and East Greenland (EGl) used in this study. Note that tusks nos. 4080, 4084 and 953 were received with ca. 5, 6 and 7 cm, respectively, cut off the bases. These missing centimetres are added to the total length of the tusks in the table. Also, it was uncertain whether the neonatal line was present in tusk no. 956 Growth layer group is abbreviated to GLG.
| ID no. |
Location |
Year |
Sex |
Body length (cm) |
Tusk length (cm) |
External tusk length (cm) |
Length of pulp cavity (cm) |
Diameter of pulp cavity (mm)a
|
Diameter of tusk (mm)a
|
Tusk weight (kg) |
Neonatal line present |
Tip of tusk present |
Base of tusk present |
No. of GLGs |
Average GLG width (mm) |
D/Lb
|
| 4080 |
Qaanaaq, NGl |
2008 |
Nac
|
Na |
91.5 |
62 |
66 |
5.5 |
26 |
0.47 |
x |
x |
– |
17 |
1.84 |
Na |
| 4084 |
Qaanaaq, NGl |
2008 |
Na |
Na |
130 |
97 |
107 |
11 |
36 |
1.16 |
x |
x |
– |
19 |
1.71 |
Na |
| 4075 |
Qaanaaq, NGl |
2008 |
F |
430 |
164 |
138 |
155 |
26 |
49 |
1.95 |
x |
x |
x |
25 |
1.57 |
0.05973 |
| 4076 |
Qaanaaq, NGl |
2008 |
M |
491 |
255 |
Na |
255 |
Na |
Na |
7.45 |
– |
– |
x |
60 |
Na |
0.10805 |
| 841 |
Fisher Isl. WGl |
2006 |
M |
456 |
179 |
145 |
165 |
26 |
58 |
3.58 |
x |
x |
x |
31 |
2.08 |
0.06914 |
| 950 |
Niaqornat, WGl |
2008 |
M |
306 |
34 |
13 |
19 |
0 |
12 |
0.06 |
x |
x |
x |
5 |
1.04 |
0.03561 |
| 951 |
Niaqornat, WGl |
2008 |
M |
358 |
109 |
81 |
92 |
6.84 |
28 |
0.79 |
x |
x |
x |
18 |
1.67 |
0.04592 |
| 953 |
Niaqornat, WGl |
2008 |
M |
471 |
201 |
160 |
171 |
18.84 |
51 |
2.77 |
x |
x |
– |
26 |
2 |
0.05763 |
| 888 |
Niaqornat, WGl |
2009 |
M |
435 |
181 |
Na |
Na |
Na |
Na |
2.33 |
x |
x |
– |
24 |
Na |
0.05850 |
| 974 |
Sc. Sound, EGl |
2010 |
M |
430 |
173 |
137 |
156 |
19 |
46 |
1.92 |
x |
x |
x |
19 |
2.85 |
0.05287 |
| 956 |
Niaqornat, WGl |
2010 |
M |
440 |
247.5 |
208.5 |
245 |
12 |
63 |
6.9 |
(x) |
x |
x |
70 |
Na |
0.1005 |
| aMeasured at the melon front. |
| bD/L ratios corrected according to regression equations. |
| cData not available. |
Tusk preparation and counting of dentinal GLGs
Total tusk length, external tusk length (portion protruding from the melon of the whale), tusk mass, tusk diameter at the melon front, total length of the pulp cavity and diameter of the pulp cavity at the melon front were measured (Table 1). Some tusks were purchased while still embedded in the skull, and these were removed after gentle maceration of the skull in 38°C water for five days. Each tusk was cut into two longitudinal sections using a jigsaw (Fig. 1). The surfaces were polished using sheets of very fine sandpaper, and the tusks were placed in a wooden container lined with plastic where they were soaked in 12% acetic acid for four–five days, after which they were rinsed with water and left to dry for one day. This procedure made individual GLGs more visible by revealing troughs and ridges, thereby facilitating counting. Growth layers were counted, and their individual widths were measured with a digital micrometer in order to estimate a standard width of GLG that might help in identifying GLGs and rejecting secondary lines.
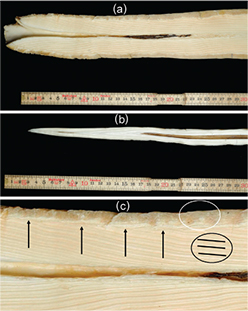
Fig. 1
(a) Root of proximal part of tusk no. 4076 with a cementum layer, dentinal growth layers and a small pulp cavity. (b) Apical tip (distal part) of tusk no. 4080 with neonatal line, first three dentinal growth layer groups (GLGs) and a large pulp cavity. (c) Detail of narwhal tusk no. 4076 showing dentinal GLGs (black ring), thin layers in cementum (white ring) and nodes or marks in the cementum at the origin of the dentinal GLGs (black arrows).
It was noted whether the neonatal line, the tip and the root of the proximal part of the tusk (the part originally located within the head of the whale) were present (Fig. 1a, b). A single, experienced tooth-reader trained in reading beluga (Delphinapterus leucas) and narwhal teeth (not tusks) was chosen to read the narwhal tusks as less-experienced tooth-readers of harp seal (Pagophilus groenlandicus) teeth have been shown to be less accurate and more negatively biased (Garde et al. 2010; Frie et al. 2011). GLGs were counted on both sides of each of the longitudinal sections, and each GLG was marked with a pencil for bookkeeping and to check for consistency in their classification. GLGs of each of the longitudinal sections were read several times, and consistent readings with the most GLGs were accepted as the best estimates. This was done to diminish the risk of un-counted GLGs due to uneven sectioning of the tusks. One GLG consisting of a small dense dark layer and a wide paler layer (Heide-Jørgensen et al. 1994) was assumed to represent one year in the whale's life, as shown for the closely related beluga (Stewart et al. 2006). Often the beginning of a GLG was marked with a node in the cementum; in cases where the GLGs were less discernible, this node could be used as guidance for determining the start of the GLGs (Fig. 1c). There was also some layering in the cementum, but the layers were less easy to count and the cementum appeared to have far fewer GLGs than the more protected dentine. Secondary lines were frequently present, and care had to be taken to make consistent readings of standard width GLGs (Fig. 1c).
Tusk growth
Growth of the narwhal tusk is shown as a plot of tusk length (cm) versus GLGs for each of the 11 narwhal tusks used in this study. One GLG is assumed to constitute one year of growth.
Eye dissection and high-performance liquid chromatography analysis
Eyes were frozen at −20°C upon collection. In the laboratory, eye lenses were dissected out of the eyes. The lens layers surrounding the nucleus were thoroughly removed, like layers of an onion, leaving only the nucleus for further analysis (Garde et al. 2007). One eye, rather than both eyes, from each whale was analysed, as no significant difference between eyes from the same individual narwhal were found in a previous study (Garde et al. 2007). Hydrolysis of samples and analysis by high-performance liquid chromatography (HPLC) were performed following the procedures of Zhao & Bada (1995) and Garde et al. (2007). Eye lens nuclei were hydrolysed in 1 ml 6 M HCl for 6 h at 100°C. An Agilent 1100 Series HPLC system (Agilent Technologies, Walbronn, Germany) was used for the chromatography and data analysis. Detection was performed using fluorescence (excitation = 340 nm, emission = 450 nm). The column was a Zorbax Eclipse XDB-C18, 4.6×150 mm, with particle size of 3.5 µm. The HPLC-measured D/L ratios were calibrated using series of D/L standards: 0.5/99.5, 1/99, 2/98, 5/95, 10/90 and 15/85. This series was run at the beginning and end of each HPLC run. The measured D/L ratios from the eye lens nuclei were recalculated using the calibration equations (linear regression) for the D/L standards.
Narwhal racemization rate and (D/L)0 value
Eye lens nuclei D/L ratios were regressed against the number of GLGs in tusks to obtain a species-specific racemization rate for narwhals (Table 1). Included in the regression were D/L ratios from two near-term foetuses (=0 years old) and 13 young narwhals classified to age based on length and month of death (age range: 0.5–2.5 years old; length range: 175–298 cm; Garde et al. 2007). The slope of the regression line equals the 2kAsp value, corresponding to twice the racemization rate. The intercept of the line corresponds to twice the (D/L)0 value. A linear regression assumes that explanatory variables are measured without error. Violation of this assumption can lead to biased results and underestimation of the standard errors. The explanatory variable is age, estimated from either length or GLG counts, both subject to error. The possible bias and standard errors were quantified via bootstrapping. Each bootstrap data set was created as follows: to the D/L ratios were added a normal random variable with mean 0 and SE equal to the residual SE from the ordinary regression assuming no error on the age estimates. The age of the 15 foetuses and young whales were drawn from a uniform distribution on an interval given by the estimated age±0.5 years, and then rounded to the nearest half-integer value. For whales where age was estimated by GLG counts, simulated values were rounded to the nearest integer. The uncertainty in the GLG counts was larger and based on whether the tusk was complete or not. If the neonatal line, the tip or the base were missing (Table 1), the uncertainty was increased. Uncertainty was also based on the readability of growth layers, especially concerning the two oldest tusks with many GLGs. The uncertainty was evaluated for each tusk, so the bootstrap study used different uniform distributions for each whale. The number of bootstrap replicates used was 10 000.
Results
Tusk measurements and GLG counts
Tusk lengths ranged from 34 to 255 cm (Table 1). Tusk diameter (mm) measured at the melon front ranged from 12 to 63 mm. Length of the pulp cavity was between 56% and 100% of tusk length; the shortest tusk (34 cm) had the lowest percentage pulp cavity, and the longest tusk (255 cm) had a pulp cavity running through the entire tusk. Tusk mass ranged from 0.06 to 7.45 kg.
Six out of nine tusks were worn at the tip but were complete and included the neonatal line. The tip of the longest tusk (ID no. 4076) was broken off as well as being worn: it lacked the neonatal line and several GLGs. The tip of the second longest tusk (ID no. 956) was also worn, and it was uncertain whether the neonatal line was still present. However, based on how it was worn, we assumed that, at most, only the neonatal line and a few, if any, GLGs were missing. To estimate the number of missing GLGs in tusk ID no. 4076, it was compared with tusk ID no. 956, which was of almost the same length and diameter. It was concluded that the missing tip of ID no. 4076 contained 10 GLGs. Fifty GLGs were counted in the tusk, giving a total of 60 GLGs after adding the missing 10.
The root of the proximal part of the tusk from whale ID no. 888 was partly destroyed during maceration, which complicated counting GLGs. The count of 24 GLGs may therefore be a minimum estimate for this tusk. The proximal parts of tusks with ID nos. 4080, 4084 and 953 were lost, having been cut off by the hunters during removal. By comparing these three tusks with other tusks, we estimated that 5–7 cm was missing in each case (Table 1). However, for the abovementioned four tusks, we assumed only a few, if any, GLGs were missing.
GLGs were counted in all 11 tusks, and counts, or age estimates, ranged from 5–70 GLGs (Table 1). Tusk growth in length for the 11 tusks ranged between 3.54 and 9.11 cm−yr with an average of 6.33 cm−yr. The two largest and oldest tusks were growing the least per year (range: 3.54–4.25 cm−yr). The remaining tusks grew between 5.38 and 7.73 cm−yr except for tusk with ID no. 974 that had the fastest growth of 9.11 cm−yr. Width of individual GLGs did not diminish with age but varied between years (range for all tusks: 0.76–3.96 mm). Tusk lengths versus number of GLGs are shown in Fig. 2.
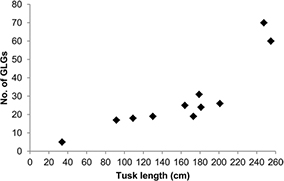
Fig. 2
Narwhal tusk length (cm) versus number of growth layer groups (GLGs). One GLG is assumed to constitute one year of tusk growth.
Standard curves and linear regression equations
The D/L standard series was used to produce two standard curves for each HPLC run. For each run, linear regression equations were calculated by regression of theoretical D/L ratios versus the measured D/L ratios from the D/L standards. The coefficient was r2=0.99 for each run. Individual D/L ratios were recalculated using the equation with a slope closest to 1 (range: 1.1295–1.2187) from each run.
Estimating the racemization rate and (D/L)0 value
The slope of the regression line (=2kAsp value) including nine whales with tusks and 15 foetuses and young whales was estimated to be 0.00233±0.000080 SE with an intercept of 0.0579±0.00179 SE (r2=0.98). The residual SE in the regression was 0.0073. The racemization rate and (D/L)0 value were, therefore, 0.00117−yr±0.000040 SE and 0.0290±0.00089 SE, respectively. For the bootstrap study, the simulated age for whales ID nos. 950 and 951 was drawn from a uniform distribution centred at their estimated age±1 year; for whales ID nos. 841, 953 and 974, an uncertainty of±3 was used; and for whale ID no. 4076, an uncertainty of ±5 was used. Finally, for whales ID nos. 4075, 888 and 956, the values given in Table 1 are minimum values, with a possible underestimate of up to five years. Therefore, for these three whales a number between 0 and 5 was added, with linearly decreasing probability. The average estimate of the slope was 0.00229±0.000089 SE, and the average intercept estimate was 0.0580±0.00185 SE (Fig. 3). The r2 was in all cases between 0.88 and 0.98. We finally estimate the racemization rate to 0.00114−yr±0.000044 and the (D/L)0 to 0.0290±0.00093.
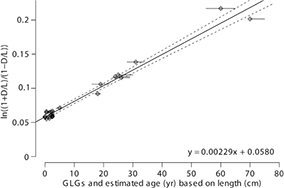
Fig. 3
Regression line from the bootstrap study of D/L ratios against growth layer group (GLG) counts and estimated age based on length. The slope of the regression line corresponds to twice the racemization rate (2kAsp) and the intercept corresponds to twice the (D/L)0 value.
Discussion
Eleven narwhal tusks and matching eyes were sampled for the purpose of estimating a species-specific racemization rate for narwhals by regression of GLGs in tusks against D/L ratios in eye lens nuclei. Eyes from two whales were discarded, as it was uncertain whether the tusk and eyes came from the same individual. Thus, samples from nine whales remained for analysis. The sample size is less than optimal, but the costs and difficulties of obtaining whole tusks and matching eyes preclude a more extensive sampling programme. Furthermore, a high incidence of broken tusks in adult males (Silverman & Dunbar 1980) impedes sampling of old and complete tusks.
The tusks in this study were not all complete (Table 1), which complicated the counting of GLGs, and there were some evident differences in the readability of the tusks that were impossible to quantify. Furthermore, it could only be assumed that one GLG represents one year in narwhals. A deposition of one GLG per year in narwhal tusks is supported by analogy to other odontocete species (Hohn 2009), and especially to the narwhal's close relative, the beluga (Stewart et al. 2006; Lockyer et al. 2007), and until proven otherwise this is the most likely deposition rate.
Tusk growth per year differed among the 11 tusks. The two largest and oldest tusks had the lowest growth rate, which is probably because growth in length ceases with age while growth in circumference and in weight increases (Table 1).
Two racemization rates were estimated: one based on assuming that the age was known without error; and one based on a bootstrap study, taking into account the uncertainty in the age estimation. Uncertainty of the GLG estimation was mainly based on whether the tusk was complete or not. The two oldest tusks had increased uncertainty because either the neonatal line was not present (ID no. 4076) or it was uncertain whether it was present or not (ID no. 956), and the neonatal line needs to be visible to obtain reliable age estimates based on GLG counts (Heide-Jørgensen et al. 1994). Another reason was that GLGs in teeth from old animals are more difficult to discern and count due to compact layering, wear of the teeth and often broken tips (Silverman & Dunbar 1980; Luque et al. 2009). The two estimated 2kAsp values are, however, almost identical. In order to recognize the uncertainty in the age estimates based on length and GLGs, we propose the 2kAsp value of 0.00229 and the (D/L)0 value of 0.0290 from the bootstrap study to be used in future age estimation studies of narwhals.
Garde et al. (2007) estimated the ages of 75 narwhals using AAR with a racemization rate estimated by linear regression using age estimates based on body length from the same 15 young narwhals used in this study and age estimates of 13 fin whales (Balaenoptera physalus) from Nerini (1983) based on counts of earplug laminations. The combined narwhal–fin whale 2kAsp value of 0.00209 is lower than the present one of 0.00229. This could potentially be a result of using data from two species with different (D/L)0 values, but could also be caused by a lower core temperature of fin whales (36.1°C, from George et al. 1999) than that of narwhals. However, the core temperature of the beluga whale, the narwhal's closest relative, has been measured to 35°C (Katsumata et al. 2006), and we assume narwhal core temperature to be similar. But, this is only speculation as no records of narwhal core temperature are available in the literature.
The 2kAsp value estimated here is specific to narwhals and should as such be a better proxy for the value in narwhals than one based on narwhal and fin whale data combined. Both the 2kAsp values from Garde et al. (2007) and the present one rely on indirect estimation of ages either through difficult GLG readings of narwhal tusks, body lengths or uncertain counts of earplug laminae in fin whales; they also rest on the assumption that one GLG is deposited per year throughout the life of the animal. Ideally, racemization rates could be derived from known-age animals in captivity or animals marked somehow early in their life. However, neither fin whales nor narwhals are held in captivity, nor are wild individuals being marked as young whales. Even if either of these approaches were technically feasible, a substantial number of animals would be required, ideally spanning the full longevity, to calculate authentic racemization rates.
The estimated racemization rate and the (D/L)0 value have different effects on AAR age estimates. AAR age estimates of older individuals are influenced mainly by the racemization rate, while age estimates of young animals are affected primarily by the (D/L)0 value (George et al. 1999). Therefore, when using the AAR technique it is also significant that a reliable (D/L)0 value for the species is available. Dentinal GLGs can be examined in cross-sections of embedded narwhal teeth, but occlusion means that only a minimum GLG count can be obtained from older whales (Hay 1980). However, the dentinal layers representing early years (before sexual maturity) are readable in embedded teeth and can apparently provide reliable age estimates of young narwhals (Hay 1980). Therefore, for future studies it is recommended that both eyes and embedded teeth be collected so that both methods of age estimation of young narwhals can be used to derive an improved estimate of the (D/L)0 value.
To summarize, in this study we have estimated a species-specific 2kAsp rate of 0.00229 and a (D/L)0 value of 0.0290 for narwhals by regression and bootstrapping of aspartic acid D/L ratios in eye lens nuclei against GLGs in tusks. These estimates are the first based entirely on narwhal data. Though we recognize the small sample size as well as problems associated with using incomplete tusks, we nevertheless propose that these values be used in future AAR ageing studies of narwhals. But we also emphasize the need for collection of embedded teeth, intact tusks and matching eyes of narwhals for further improving the (D/L)0 and 2kAsp estimates obtained in this study and for examining both methods of age estimation.
Acknowledgements
Thanks to Jeppe Møhl for cutting and preparing the narwhal tusks, and to Nynne Hjort Nielsen, Marianne Andersen and Kirsten Andersen for assistance with eye dissections and HPLC analysis. Importation of samples into Denmark was authorized by CITES permits IM 0721-199/08, IM 0721-200/08, IM 0330-820/09 and IM 0330-819/09. Funding for this study was obtained from the Greenland Institute of Natural Resources and the Danish Cooperation for the Environment in the Arctic.
References
Bada
J.L.,
Brown
S.
&
Masters
P.M. 1980.
Age determination of marine mammals based on aspartic acid racemization in the teeth and lens nucleus. Report of the International Whaling Commission Special Issue 3,
113–118.
Bada
J.L.,
Mitchell
E.
&
Kemper
B. 1983.
Aspartic acid racemization in narwhal teeth. Nature 303,
418–420.
[Crossref]
Dietz
R.,
Heide-Jørgensen
M.P.,
Härkönen
T.,
Teilmann
J.
&
Valentin
N. 1991.
Age determination of European harbour seals Phoca vitulina L. Sarsia 76,
17–21.
Frie
A.K.,
Fagerheim
K.,
Hammill
M.O.,
Kapel
F.O.,
Lockyer
C.,
Stenson
G.B.,
Rosing-Asvid
A.
&
Svetochev
V. 2011.
Error patterns in age estimation of harp seals (Pagophilus groenlandicus): results from a transatlantic, image-based, blind-reading experiment using known-age teeth. ICES Journal of Marine Science 68,
1942–1953.
[Crossref]
Garde
E. 2011. Past and present population dynamics of narwhals Monodon monoceros. PhD thesis,
Natural History Museum of Denmark, University of Copenhagen.
Garde
E.,
Frie
A.K.,
Dunshea
G.,
Hansen
S.H.,
Kovacs
K.M.
&
Lydersen
C. 2010.
Harp seal ageing techniques—teeth, aspartic acid racemization, and telomere sequence analysis. Journal of Mammalogy 91,
1365–1374.
[Crossref]
Garde
E.,
Heide-Jørgensen
M.P.,
Hansen
S.H.,
Nachman
G.
&
Forchammer
M.C. 2007.
Age-specific growth and remarkable longevity in narwhals (Monodon monoceros) from West Greenland as estimated by aspartic acid racemization. Journal of Mammalogy 88,
49–58.
[Crossref]
George
J.C.,
Bada
J.L.,
Zeh
J.,
Scott
L.,
Brown
S.E.,
O'Hara
T.
&
Suydam
R. 1999.
Age and growth estimates of bowhead whales (Balaena mysticetus) via aspartic acid racemization. Canadian Journal of Zoology 77,
571–580.
Hay
K.A. 1980.
Age determination of the narwhal, Monodon monoceros L. Report of the International Whaling Commission Special Issue 3,
119–132.
Hay
K.A.
&
Mansfield
A.W.
1989.
Narwhal—Monodon monoceros Linnaeus, 1758.
In S.H. Ridgway & R. Harrison (eds.): Handbook of marine mammals. Pp. 145–176. London: Academic Press.
Heide-Jørgensen
M.P.,
Dietz
R.,
Laidre
K.L.,
Richard
P.,
Orr
J.
&
Schmidt
H.C. 2003.
The migratory behaviour of narwhals (Monodon monoceros.) Canadian Journal of Zoology 81,
1298–1305.
[Crossref]
Heide-Jørgensen
M.P.,
Jensen
J.,
Larsen
A.,
Teilmann
J.
&
Neurohr
B. 1994.
Age estimation of white whales (Delphinapterus leucas) from Greenland. Meddelelser om Grønland, Bioscience 39,
187–193.
Heide-Jørgensen
M.P.,
Laidre
K.L.,
Burt
M.L.,
Borchers
D.L.,
Marques
T.A.,
Hansen
R.G.,
Rasmussen
M.
&
Fossette
S. 2010.
Abundance of narwhals (Monodon monoceros) on the hunting grounds in Greenland. Journal of Mammalogy 91,
1135–1151.
[Crossref]
Hohn
A.A.
2009.
Age estimation.
In W.F. Perrin et al. (eds.): Encyclopedia of marine mammals. 2nd edn. Pp. 11–17. San Diego: Academic Press.
Katsumata
E.,
Furuta
C.,
Katsumata
H.,
Watanabe
G.
&
Taya
K. 2006.
Basal body temperature method for detecting ovarian cycle in captive beluga (Delphinapterus leucas.) Journal of Reproduction and Development 52,
59–63.
[Crossref]
Kingsley
M. 1989.
Population dynamics of the narwhal Monodon monoceros: an initial assessment (Odontoceti: Monodontidae). Journal of Zoology 219,
201–208.
[Crossref]
Lockyer
C.,
Hohn
A.A.,
Doidge
D.W.,
Heide-Jørgensen
M.P.
&
Suydam
R. 2007.
Age determination in belugas (Delphinapterus leucas)—a quest for validation of dentinal layering. Aquatic Mammals 33,
293–304.
[Crossref]
Luque
P.L.,
Pierce
G.J.,
Learmonth
J.A.,
Santos
M.B.,
Ieno
E.,
Lopéz
A.,
Reid
R.J.,
Rogan
E.,
Gonzáles
A.F.,
Boon
J.,
Law
R.J.
&
Lockyer
C.H. 2009.
Dentinal anomalies in teeth of harbour porpoises (Phocoena phocoena) from Scottish waters: are they linked to sexual maturation and environmental events? Journal of the Marine Biological Association of the United Kingdom 89,
893–902.
[Crossref]
Masters
P.M.,
Bada
J.L.
&
Zigler
J.S. 1977.
Aspartic acid racemization in the human lens during ageing and in cataract formation. Nature 268,
71–73.
[Crossref]
NAMMCO (North Atlantic Marine Mammal Commission) 2009. Annual report 2009.
Tromsø,
Norway: North Atlantic Marine Mammal Commission.
Nerini
M.K. 1983.
Age determination of fin whales (Balaenoptera physalus) based upon aspartic acid racemization in the lens nucleus. Report of the International Whaling Commission 33,
447–448.
Olsen
E.
&
Sunde
J. 2002.
Age determination of minke whales (Balaenoptera acutorostrata) using the aspartic acid racemization technique. Sarsia 87,
1–8.
[Crossref]
Reeves
R.R.
&
Heide-Jørgensen
M.P. 1994.
Commercial aspects of the exploitation of narwhals (Monodon monoceros) in Greenland, with emphasis on tusk exports. Meddelelser om Grønland, Bioscience 39,
119–134.
Richard
P.R.,
Laake
J.L.,
Hobbs
R.C.,
Heide-Jørgensen
M.P.,
Asselin
N.C.
&
Cleator
H. 2010.
Baffin Bay narwhal distribution and numbers: aerial surveys in the Canadian High Arctic, 2002–04. Arctic 63,
85–99.
Rosa
C.,
Zeh
J.,
George
J.C.,
Botta
O.,
Zauscher
M.,
Bada
J.
&
O'Hara, T.M.
2012. Age estimates based on aspartic acid racemization for bowhead whales (Balaena mysticetus) harvested in 1998–2000 and the relationship between racemization rate and body temperature. Marine Mammal Science, doi: 10.1111/j.1748-7692.2012.00593.x.
[Crossref]
Silverman
H.B.
&
Dunbar
M.J. 1980.
Aggressive tusk use by the narwhal (Monodon monoceros L.). Nature 284,
57–58.
[Crossref]
Stewart
R.E.A.,
Campana
S.E.,
Jones
C.M.
&
Stewart
B.E. 2006.
Bomb radiocarbon dating calibrates beluga (Delphinapterus leucas) age estimates. Canadian Journal of Zoology 84,
1840–1852.
[Crossref]
Zhao
M.
&
Bada
J.L. 1995.
Determination of α-dialkylamino acids and their enantiomers in geological samples by high-performance liquid chromatography after derivatization with a chiral adduct of o-phthaldialdehyde. Journal of Chromatography A 690,
55–63.
[Crossref]