RESEARCH/REVIEW ARTICLE
High-temperature optima phosphatases from the cold-tolerant Arctic fungus Penicillium citrinum
Puja Gawas-Sakhalkar, Shiv Mohan Singh, Simantini Naik & Rasik Ravindra
National Centre for Antarctic and Ocean Research, Headland Sada, Goa 403 804, India
Abstract
Fifty-six fungal isolates from Arctic soils were subjected to primary screening for their ability to solubilize insoluble inorganic phosphate. Nine of the isolates were further analysed quantitatively for phosphatase production using para-nitrophenylphosphate as substrate. Amongst these, a cold-tolerant fungus, Penicillium citrinum strain PG162 was found to be the best producer of intracellular acid phosphatase. Further characterization of the enzyme showed that it is most active in the temperature range of 40–60°C and pH range of 4.2–4.8. The dried enzyme extract is stable at a temperature of up to 50°C for at least 1 h. Its activity is affected by presence of metal ions. Sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS–PAGE) analysis suggests the molecular weight of the enzyme to be between 20 and 29 kDa. The present study is important with respect to our understanding of the kind of enzymatic reactions that take place in the polar microbes, and the extent to which their activity is sustained.
Keywords
Acid phosphatase; enzyme; para-nitrophenylphosphate; Pikovskaya medium; psychrotolerant; SDS–PAGE.
Correspondence
Shiv Mohan Singh, National Centre for Antarctic and Ocean Research, Headland
Sada, Goa 403 804, India. E-mail: smsingh@ncaor.org
(Published: 9 August 2012)
Polar Research 2012. © 2012 P. Gawas-Sakhalkar et al. This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial 3.0 Unported License (http://creativecommons.org/licenses/by-nc/3.0/), permitting all non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Citation: Polar Research 2012, 31, 11105, http://dx.doi.org/10.3402/polar.v31i0.11105
Phosphatases or phosphoric monoester hydrolases (EC 3.1.3) are a group of enzymes, occurring ubiquitously in nature, that non-specifically hydrolyse a wide variety of phosphate esters and anhydrides of phosphoric acid to produce inorganic phosphate (Holander 1971). Broadly, these enzymes can be classified into two categories: alkaline phosphatase, EC 3.1.3.1—these are enzymes that act at an optimum pH >8); and acid phosphatase, EC 3.1.3.2—enzymes that act at an optimum pH <6 (Guimarães et al. 2001).
In soil microorganisms phosphatases play a major role in mobilizing organically bound phosphate in the soils and are frequently used as a marker of microbial activity in soil ecology studies (Kuperman & Carreiro 1997). Secretion of phosphatase enzymes occurs in response to both phosphate starvation and environmental pH signalling, instigating the microbes to utilize phosphorus containing substrates (Caddick et al. 1986).
There are several reports of phosphatase activity shown by microbes in tropical soils (e.g., Nopparat et al. 2007; Nenwani et al. 2010). There are fewer such records from temperate regions (Turner & Haygarth 2005) and from the Arctic such activity has only recently been demonstrated (Singh et al. 2011). Phosphatase activity directly in soils has, however, been reported earlier from the Arctic tundra (Neal 1990) and High-Arctic glaciers (Stibal et al. 2009).
Fungi are reported to solubilize phosphorus by production of organic acids and are known to have a higher solubilizing efficiency than bacteria (Nenwani et al. 2010). Efforts have been made to encapsulate phosphate solubilizing fungi for use in agriculture and industry (Vassileva et al. 1998). Microfungal genera such as Aspergillus (Nozawa et al. 1998), Penicillium (Yoshida et al. 1989; Haas et al. 1991), Fusarium (Yoshida & Tamiya 1971) and Neurospora (Nahas & Rossi 1984) as well as ectomycorrhizal macromycetes (Antibus et al. 1986; Sharma et al. 2010), are known to produce phosphatase. Cold-tolerant strains of ectomycorrhizal Hebeloma spp. are also reported to produce cold active acid phosphatases (Tibbett et al. 1998).
The present study was carried out with the aim of screening and characterizing the Arctic soil fungi for phosphatase production. Good phosphatase producers from cold-tolerant microbes have the potential for use in biotechnology-based industries and in low-temperature farming activities in the cold regions of the world.
Materials and methods
Organisms
Fifty-six isolates of the soil microflora were tested for their ability to solubilize insoluble inorganic phosphate. These include Aureobasidium sp. (1), Aureobasidium pullulans (2), Botrytis verrucosa (1), Chrysosporium pannorum (2), Cladosporium chlorocephalum (1), C. cladosporioides (1), Fusarium oxysporum (1), Microdochium sp. (2), Mortierella sp. (1), Mortierella alpina (4), M. schmuckeri (1), M. simplex (1), Mucor hiemalis (3), Penicillium sp. (1), Penicillium citrinum (8), P. frequentans (1), P. rugulosum (1), Phialophora sp. (5), Pithomyces chartarum (1), Trichosporiella cerebriformis (1) and non-sporulating morphotypes (NSMs; 7). Figures in parentheses indicate number of strains.
Primary screening
Peripheral discs of 7–10-day old cultures, grown on malt extract agar, were centrally inoculated on Pikovskaya agar medium containing (g/l): yeast extract 0.5, dextrose 10, Ca3(PO4)2 5, (NH4)2SO4 0.5, KCl 0.2, MgSO4·7H2O 0.1, MnSO4·7H2O 0.0001, FeSO4·7H2O 0.0001 and Agar 18 (Pikovskaya 1948), maintained at pH 4.8 (for acid phosphatase) and pH 9.8 (for alkaline phosphatase). The petriplates were incubated for 10 days at 15°C. A clear halo around the faintly turbid medium indicated positive activity. On the basis of the extent of the halo the isolates were qualitatively sorted into four groups: good activity, moderate activity, low activity and no activity (Table 1).
Table 1 Preliminary screening of fungal isolates for their ability to solubilize insoluble inorganic phosphate. Phosphase activity is rated as follows: good activity (+ + +), moderate activity (+ + ), low activity (+) and no activity (−).
| Fungal name |
Phosphatase activity |
Fungal name |
Phosphatase activity |
| Aureobasidium pullulans PG134 |
− |
Penicillium citrinum PG191 |
− |
| Aureobasidium pullulans PG135 |
− |
Penicillium frequentans PG185 |
+ + + |
| Aureobasidium sp. PG129 |
− |
Penicillium rugulosum PG49 |
+ + + |
| Botrytis verrucosa PG43 |
+ + + |
Penicillium sp. PG186 |
− |
| Chrysosporium pannorum PG140 |
+ + |
Phialophora sp. PG30 |
− |
| Chrysosporium pannorum PG47 |
+ + + |
Phialophora sp. PG44 |
+ |
| Cladosporium chlorocephalum PG187 |
− |
Phialophora sp. PG81 |
+ |
| Cladosporium cladosporioides PG87 |
− |
Phialophora sp. PG133 |
+ |
| Fusarium oxysporum PG28 |
+ + |
Phialophora sp. PG42 |
− |
| Microdochium sp. PG207 |
+ + |
Pithomyces chartatum PG46 |
+ + |
| Microdochium sp. PG209 |
+ + |
Trichosporiella cerebriformis PG48 |
+ |
| Mortierella alpina PG188 |
− |
NSM PG83 |
− |
| Mortierella alpina PG204 |
− |
NSM PG84 |
− |
| Mortierella alpina PG40 |
+ + + |
NSM PG106 |
− |
| Mortierella alpina PG41 |
− |
NSM PG107 |
− |
| Mortierella schmuckeri PG45 |
+ |
NSM PG113 |
− |
| Mortierella simplex PG26 |
+ |
NSM PG128 |
+ + |
| Mortierella sp. PG205 |
− |
NSM PG138 |
− |
| Mucor hiemalis PG10 |
+ + |
NSM PG139 |
− |
| Mucor hiemalis PG151 |
+ |
NSM PG147 |
− |
| Mucor hiemalis PG91 |
+ + |
NSM PG149 |
− |
| Penicillium citrinum PG31 |
+ + |
NSM PG152 |
− |
| Penicillium citrinum PG32 |
+ + + |
NSM PG155 |
− |
| Penicillium citrinum PG79 |
+ + + |
NSM PG159 |
− |
| Penicillium citrinum PG130 |
− |
NSM PG160 |
− |
| Penicillium citrinum PG162 |
+ + + |
NSM PG192 |
− |
| Penicillium citrinum PG189 |
− |
NSM PG206 |
− |
| Penicillium citrinum PG190 |
+ + + |
NSM PG208 |
+ |
Enzyme quantification
Isolates that exhibited good activity during primary screening were subjected to phosphomonoesterase quantification using para-nitrophenyl phosphate (p-NPP), an analogue of phosphate monoester substrate. Mycelial discs of the isolates were inoculated in Pikovskaya broth and incubated at 15°C and 160 rpm for 7 days. The cultures were harvested by filtration.
The mycelium was collected, rinsed twice with sterile distilled water, lyophilized and weighed. Both culture filtrate and mycelia were subjected to acid and alkaline phosphatase assay. The dried mycelium was pounded using a mortar and pestle with slow addition of 20 ml of ice-cold citrate buffer (pH 4.8) and glycine buffer (pH 10.4) for acid and alkaline phosphatase assay, respectively. The solution was mixed without allowing any bubble formation and centrifuged at 4°C at 8000 rpm for 10 min. The supernatant was collected and used as an enzyme source.
Acid phosphatase assay was carried out according to Bergmeyer (1974), with slight modification. Five hundred microlitre of 90 mM citrate buffer was added to equal volume of 15.2 mM p-NPP (prepared in the same buffer) and equilibrated at 40°C for 5 min. Enzyme (100 µl) was added to the reaction mixture and the tubes were incubated for 15 min at 40°C. The reaction was terminated by addition of 4 ml 100 mM NaOH. Reaction blanks were maintained by substituting enzyme with an equivalent volume of buffer. Absorbance was measured at 410 nm against blank using a UV–Vis spectrophotometer (Analytik Jena, Jena, Germany). The amount of p-nitrophenol released was calculated using the millimolar extinction coefficient of p-nitrophenol at 410 nm. One unit will hydrolyse 1.0 µmole of p-nitrophenyl phosphate per minute under given conditions of temperature, pH, incubation time and enzyme dilution.
Alkaline phosphatase assay was carried out similarly but with the following variation. The buffer used was 100 mM glycine buffer (pH 10.4) supplemented with 1 mM MgCl2 and the termination solution was 10 mM NaOH.
Growth rate of the isolate was measured by growing the culture onto potato dextrose agar medium at 4, 15 and 28°C.
Activity at different pH and temperatures
Optimum temperature for activity of the intracellular acid phosphatase enzyme was determined by incubating the enzyme with buffered p-NPP at different temperatures, viz. 5, 10, 20, 30, 40, 50, 60 and 70°C. Similarly, optimum pH was determined by using 100 mM citrate buffer at various pH values: 3.0, 3.6, 4.2, 4.8, 5.4 and 6.0.
Activity in presence of metal ions and enzyme stability at different temperatures
Effect of metal ions and ethylenediaminetetraacetic acid (EDTA) on the activity of the enzyme was determined by incubating the enzyme with 1 mM solutions of Fe2(SO4)3, FeSO4·7H2O, CoCl2·6H2O, CaCl2·2H2O, CuSO4, ZnSO4·7H2O, MoO3, MnSO4·H2O, MgSO4·7H2O and Na-salt of EDTA.
Stability of the enzyme at different temperatures was determined by incubating the lyophilized enzyme extracts at various temperatures—20, 30, 40, 50, 60 and 80°C—for 1 h and later subjecting it to p-NPP assay at 40°C.
Electrophoresis
Intracellular acid phosphatase enzyme from P. citrinum strain PG162 was subjected to 15% sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS–PAGE) and stained with Coomassie brilliant blue R250 dye to visualize bands (Makowski & Ramsby 1997).
Statistical analyses
The data was subjected to analyses of variance (ANOVA) using the PAST software (Hammer et al. 2001).
Results and discussion
Of the 56 isolates screened, 26 showed positive activity while 30 showed no activity. Amongst those that showed positive activity, nine exhibited good, nine moderate and eight showed mild activity. Most NSMs showed no activity (Table 1). The nine isolates showing good activity were Botrytis verrucosa (1), Chrysosporium pannorum (1), Mortierella alpina (1), Penicillium citrinum (4), P. frequentans (1) and P. rugulosum (1).
Results for intracellular and extracellular alkaline and acid phosphatases activities for these nine isolates are given in Table 2. Almost all screened cultures produced intracellular phosphatases at varying levels. The best producer amongst these was P. citrinum strain PG162. This study is the first report of acid phosphatase production by a cold-tolerant P. citrinum strain, although other non-psychrotrophic species of Penicillium (P. funiculosum and P. chrysogenum) are already known to produce the enzyme (Yoshida et al. 1989; Haas et al. 1991). Cold-tolerant strains of a bacterium (Pseudomonas sp.) and fungus (Aspergillus niger) are also known to solubilize phosphate (Trivedi & Pandey 2007; Singh et al. 2011).
Table 2 Quantification of intracellular and extracellular alkaline and acid phosphatases. Data in the same column followed by a different alphabet letter are significantly different according to the one-way ANOVA test (P<0.05). Boldface indicates the highest activity.
|
Intracellular (U/g mdw) |
Extracellular (U/ml) |
| Isolate |
Acid phosphatase |
Alkaline phosphatase |
Acid phosphatase |
Alkaline phosphatase |
| Penicillium citrinum PG32 |
2.13±0.08a
|
0.50± 0.05a
|
0.01±0.0 |
0 |
| Mortierella alpina PG40 |
0.07±0.02b
|
0.01± 0.0b
|
0.01±0.0 |
0 |
| Botrytis verrucosa PG43 |
0.12±0.06b
|
0.01± 0.0b
|
0.01±0.0 |
0 |
| Chrysosporium pannorum PG47 |
0.35±0.1b
|
0.00±0.0b
|
0.01±0.0 |
0 |
| Penicillium rugulosum PG49 |
10.23±0.6c
|
0.83±0.04c
|
0.02±0.0 |
0 |
| Penicillium citrinum PG79 |
0.71±0.04b
|
0.00±0.0b
|
0.01±0.0 |
0 |
| Penicillium citrinum PG162 |
11.48±0.38d
|
0.47±0.03d
|
0.02±0.0 |
0 |
| Penicillium frequentans PG185 |
3.42±0.06e
|
0.53±0.0d
|
0.02±0.0 |
0 |
| Penicillium citrinum PG190 |
4.18±0.28f
|
0.11±0.01e
|
0.03±0.0 |
0 |
After 15 days incubation, the growth of the culture P. citrinum PG162 was observed to be about 21–26 mm at 4°C, 33–35 mm at 15°C. The culture ceased to grow at 28°C. Temperature optima of the phosphatase enzyme extracted from the culture was between 40 and 60°C, with highest activity at 60°C (Fig. 1a). Few other cold-adapted microbes are reported to produce enzymes with temperature optima in the mesophilic range. β-galactosidase isolated from Antarctic Bacillus sp. had a temperature optima of 40°C while protease from Antarctic Stenotrophomonas maltophila and Pseudoalteromonas sp. had an optimum temperature range between 55 and 60°C and between 35 and 60°C, respectively (Dhaked et al. 2005; Vázquez et al. 2005; Vázquez et al. 2008). These observations indicate that the optimal physiological state of these organisms is reached at temperatures well below the optimal temperature required for the activity of the produced enzymes. Vázquez et al. (2008) and Margesin & Schinner (1999) considered this to be an adaptation of microbes living in a cold environment relying more on increasing the amount of high-specific-activity enzymes produced rather than the ability of the enzymes to work efficiently at low temperature. Bölter (2004) has reviewed in detail the ecophysiological behaviour, as a part of survival strategies, in such cold-adapted microbes.
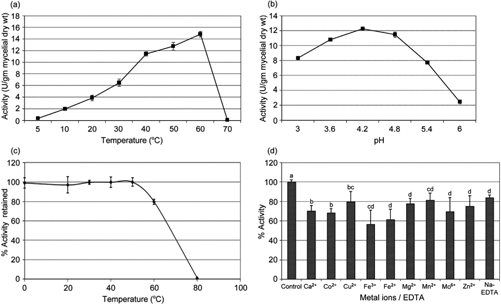
Fig. 1
Enzyme properties: (a) effect of temperature on phosphatase activity, (b) effect of pH on phosphatase activity, (c) effect of temperature on phosphatase enzyme stability, (d) effect of metal ions and ethylenediaminetetraacetic acid (EDTA) on phosphatase activity. At the edge of each bar, a different letter indicates significant difference in the values according to the one-way ANOVA test (P<0.05).
The culture showed optimal phosphatase activity at pH 4.2 although the difference in activity at pH 4.2 and 4.8 was statistically insignificant (Fig. 1b). The dried enzyme was stable for at least 1 h at temperatures up to 50°C but lost activity with further increase in temperature (Fig. 1c).
Metal ions are known to affect the activity and stability of enzymes (Coleman et al. 1983). The activity of acid phosphatase enzyme was affected by all the metal ions studied with Fe3 + and Fe2 + ions showing maximum effect and Mn2 + ions the least (Fig. 1d). All the values were lower than the control and the differences were statistically significant. Sodium salt of EDTA also lowered the enzyme activity, suggesting that some metal ions are involved in the proper functioning of the enzyme which, when chelated, affects the activity.
The SDS–PAGE band profile of the enzyme suggests its molecular weight to be between 20 and 29 kDa. The length of the band also suggests the possibility of two or more isozymes of the enzyme. Further studies are being conducted to explore this.
This report of intracellular acid phosphatase from P. citrinum and its characterization adds to our understanding of the nature of enzymes produced by polar cold-tolerant fungi and the extent to which its activity is sustained.
Acknowledgements
We are indebted to The Secretary, Ministry of Earth Sciences, Government of India, for financial support and facilities. This is National Centre for Antarctic and Ocean Research contribution no. 11/2012.
References
Antibus
R.K.,
Kroehler
C.J. &
Linkins
A.E. 1986.
The effects of external pH temperature and substrate concentration on acid phosphatases activity of ectomycorrhizal fungi.
Canadian Journal of Botany
64,
2383–2387.
[Crossref]
Bergmeyer
H.U. 1974. Methods of enzymatic analysis. Vol.
1.
New York: Academic Press.
Bölter
M.
2004.
Ecophysiology of psychrophilic and psychrotolerant microorganisms.
Cell and Molecular Biology
50,
563–573.
Caddick
M.X.,
Brownlee
A.G. &
Arst
H.N.
1986.
Regulation of gene expression by pH of the growth medium in Aspergillus nidulans.
Molecular and General Genetics 203,
346–353.
[Crossref]
Coleman
J.E.,
Nakamura
K. &
Chlebowski
J.F.
1983.
65Zn(II), 115Cd(II), 60Co(II), and Mg(II) binding to alkaline phosphatase of Escherichia coli. Structural and functional effects.
The Journal of Biological Chemistry 258,
386–395.
Dhaked
R.K.,
Alam
S.I. &
Singh
L.
2005.
Characterization of β-galactosidase from an Antarctic Bacillus sp.
Indian Journal of Biotechnology 4,
227–231.
Guimarães
L.H.S.,
Jorge
J.A.,
Terenzi
H.F. &
Polizeli M.L.T.M.
2001.
Thermostable conidial and mycelial alkaline phosphatases from thermophilic fungus Scytalidium thermophilum.
Journal of Industrial Microbiology and Biotechnology 27,
265–270.
[Crossref]
Haas
H.,
Redl
B.,
Leitner
E. &
Stoffler G.
1991.
Penicillium chrysogenum extracellular acid phosphatase: purification and biochemical characterization.
Biochimica et Biophysica Acta 1074,
392–397.
[Crossref]
Hammer
Ø.,
Harper
D.A.T.
&
Ryan
P.D.
2001. PAST: paleontological statistics software package for education and data analysis.
Palaeontologia Electronica 4, article no. 4.
Holander
V.P. 1971.
Acid phosphatase.
In P.D. Boyer (ed.): Enzymes. Pp. 450–498. New York: Academic Press.
Kuperman
R.G. &
Carreiro
M.M.
1997.
Soil heavy metal concentrations, microbial biomass and enzyme activities in a contaminated grassland ecosystem.
Soil Biology and Biochemistry 29,
179–190.
[Crossref]
Makowski
G.S. &
Ramsby
M.L. 1997.
Protein molecular weight determination by sodium dodecyl sulfate polyacrylamide gel electrophoresis.
In T.E. Creighton (ed.): Protein structure. A practical approach. Pp. 1–27. Oxford: Oxford University Press.
Margesin
R.
&
Schinner
F. (eds.) 1999. Cold adapted organisms: ecology, physiology, enzymology and molecular biology.
Berlin: Springer.
Nahas
E. &
Rossi
A. 1984.
Properties of a repressible alkaline phosphatase secreted by the wild-type strain 74A of Neurospora crassa.
Phytochemistry 23,
507–510.
[Crossref]
Neal
J.L. 1990.
Phosphatase enzyme activity at subzero temperatures in Arctic tundra soils.
Soil Biology & Biochemistry 22,
883–884.
[Crossref]
Nenwani
V.,
Doshi
P.,
Saha
T. &
Rajkumar
S. 2010.
Isolation and characterization of a fungal isolate for phosphate solubilization and plant growth promoting activity.
Journal of Yeast and Fungal Research 1,
9–14.
Nopparat
C.,
Jatupornpipat
M. &
Rittiboon
A. 2007.
Isolation of phosphate solubilizing fungi in soil from Kanchanaburi, Thailand.
KMITL Science and Technology Journal 7,
137–146.
Nozawa
S.R.,
Maccheroni
W.,
Stabeli
R.G.,
Thedei
G. &
Rossi
A. 1998.
Purification and properties of Pi repressible acid phosphatases from Aspergillus nidulans.
Phytochemistry 49,
1517–1523.
[Crossref]
Pikovskaya
R.I. 1948.
Mobilization of phosphorus in soil connection with the vital activity of some microbial species.
Mikrobiologia 17,
362–370.
Sharma
R.,
Baghel
R.K. &
Pandey
A.K. 2010.
Dynamics of acid phosphatase production of the ectomycorrhizal mushroom Cantharellus tropicalis.
African Journal of Microbiology Research 4,
2072–2078.
Singh
S.M.,
Yadav
L.,
Singh
S.K.,
Singh
P.,
Singh
P.N.
&
Ravindra
R.
2011. Phosphate solubilizing ability of two Arctic Aspergillus niger strains.
Polar Research 30, article no. 7283, doi: 10.3402/polar.v30i0.7283.
[Crossref]
Stibal
M.,
Anesio
A.M.,
Blues
C.J.D. &
Tranter
M. 2009.
Phosphatase activity and organic phosphorus turnover on a High Arctic glacier.
Biogeosciences 6,
913–922.
[Crossref]
Tibbett
M.,
Grantham
K.,
Sanders
F.E. &
Cairney
J.W.G. 1998.
Induction of cold active acid phosphomonoesterase activity at low temperature in psychrotrophic ectomycorrhizal Hebeloma spp.
Mycological Research 102,
1533–1539.
[Crossref]
Trivedi
P. &
Pandey
A. 2007.
Low temperature phosphate solubilization and plant growth promotion by psychrotrophic bacteria, isolated from Indian Himalayan region.
Research Journal of Microbiology 2,
454–461.
[Crossref]
Turner
B.L. &
Haygarth
P.M. 2005.
Phosphatase activity in temperate pasture soils: potential regulation of labile organic phosphorus turnover by phosphodiesterase activity.
Science of the Total Environment 344,
27–36.
[Crossref]
Vassileva
M.,
Azcon
R.,
Barea
J.M. &
Vassilev
N. 1998.
Application of an encapsulated filamentous fungus in solubilization of inorganic phosphate.
Journal of Biotechnology 63,
67–72.
[Crossref]
Vázquez
S.C.,
Hernández
E. &
Cormack
W.P.M. 2008.
Extracellular proteases from the Antarctic marine Pseudoalteromonas sp. P96-47 strain.
Revista Argentina de Microbiología 40,
63–71.
Vázquez
S.,
Ruberto
L. &
Cormack
W.M. 2005.
Properties of extracellular proteases from three psychrotolerant Stenotrophomonas maltophilia isolated from Antarctic soil.
Polar Biology 28,
319–325.
[Crossref]
Yoshida
H. &
Tamiya
N. 1971.
Acid phosphatases from Fusarium moniliforme. Purification and enzymatic properties.
The Journal of Biochemistry 69,
525–534.
Yoshida
H.,
Oikawa
S.,
Ikeda
M. &
Reese
E.T. 1989.
A novel acid phosphatase excreted by Penicillium funiculosum that hydrolyses both phosphodiesters and phosphomonoesters with aryl leaving groups.
The Journal of Biochemistry 105,
794–798.