RESEARCH/REVIEW ARTICLE
Low genetic diversity in Antarctic populations of the lichen-forming ascomycete Cetraria aculeata and its photobiont
Stephanie Domaschke,1,2 Fernando Fernández-Mendoza,1,2 Miguel A. García,3 María P. Martín3 & Christian Printzen1
1 Department of Botany and Molecular Evolution, Senckenberg Research Institute, Senckenberganlage 25, DE-60325 Frankfurt am Main, Germany
2 Biodiversity and Climate Research Center, Senckenberganlage 25, DE-60325 Frankfurt am Main, Germany
3 Real Jardín Botánico, RJB-CSIC, Plaza de Murillo 2, ES-28014 Madrid, Spain
Abstract
Lichens, symbiotic associations of fungi (mycobionts) and green algae or cyanobacteria (photobionts), are poikilohydric organisms that are particularly well adapted to withstand adverse environmental conditions. Terrestrial ecosystems of the Antarctic are therefore largely dominated by lichens. The effects of global climate change are especially pronounced in the maritime Antarctic and it may be assumed that the lichen vegetation will profoundly change in the future. The genetic diversity of populations is closely correlated to their ability to adapt to changing environmental conditions and to their future evolutionary potential. In this study, we present evidence for low genetic diversity in Antarctic mycobiont and photobiont populations of the widespread lichen Cetraria aculeata. We compared between 110 and 219 DNA sequences from each of three gene loci for each symbiont. A total of 222 individuals from three Antarctic and nine antiboreal, temperate and Arctic populations were investigated. The mycobiont diversity is highest in Arctic populations, while the photobionts are most diverse in temperate regions. Photobiont diversity decreases significantly towards the Antarctic but less markedly towards the Arctic, indicating that ecological factors play a minor role in determining the diversity of Antarctic photobiont populations. Richness estimators calculated for the four geographical regions suggest that the low genetic diversity of Antarctic populations is not a sampling artefact. Cetraria aculeata appears to have diversified in the Arctic and subsequently expanded its range into the Southern Hemisphere. The reduced genetic diversity in the Antarctic is most likely due to founder effects during long-distance colonization.
To access the supplementary material to this article please see Supplementary files under Article Tools online.
Keywords
Genetic diversity; lichens; Cetraria aculeata; Trebouxia jamesii; polar lichens; global change.
Correspondence
Stephanie Domaschke, Department of Botany and Molecular Evolution, Senckenberg Research Institute, Senckenberganlage 25, DE-60325 Frankfurt am Main, Germany. E-mail: sdomaschke@senckenberg.de
(Published: 27 March 2012)
Polar Research 2012. © 2012 S. Domaschke et al. This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial 3.0 Unported License (http://creativecommons.org/licenses/by-nc/3.0/), permitting all non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Citation: Polar Research 2012, 31, 17353, DOI: 10.3402/polar.v31i0.17353
The environmental conditions of the Antarctic are among the most adverse on Earth and are generally characterized by low mean annual temperatures, high wind velocities, extreme drought and extended periods of darkness. The effects of global climate change are especially pronounced in parts of the Antarctic (Turner et al. 2005). Air temperature in the maritime Antarctic has steadily increased within the last years (Smith & Stammerjohn 1996; Turner et al. 2005). On the western Antarctic Peninsula a temperature increase of more than 2.5 K has been observed over the last 50 years. The overall effect of such a temperature increase on terrestrial Antarctic organisms could be beneficial. For example, glacial melting will increase the availability of terrestrial habitats and therefore might initiate population growth and the spread of species to locations that are now inaccessible. On the other hand, north–south movements of air masses (Wynn-Williams 1991) offer opportunities for long distance dispersal from more temperate areas, as demonstrated by Smith (1993), among others. Consequently, competition with invading species from temperate regions could just as well lead to increased stress, reduced population sizes and extinction of Antarctic species. The effect of air temperature increase on the species composition of terrestrial Antarctic ecosystems has already been demonstrated by Smith (1991, 1994), Cornelissen et al. (2001) and others. How Antarctic organisms will cope with these environmental changes depends largely on their adaptive potential, which in turn depends to a large degree on the genetic variability of populations.
The genetic variability of species is shaped by several forces, including demographic history, spatial distribution and isolation of populations and natural selection. Population size bottlenecks, including founder events during colonization of new habitats, as well as fragmentation and long-standing geographical isolation will generally reduce the genetic diversity of populations by random genetic drift (Nei et al. 1975). Similarly, strong natural selection can reduce genetic diversity by removing poorly adapted genotypes from the population. This loss of genetic diversity through genetic drift or selective sweeps is balanced by random mutation or, on a shorter time scale, by gene flow through migration or dispersal from surrounding populations (Ingvarsson 2001). Reduced genetic diversity has consequences for the fitness and the evolutionary potential of a species (Fisher 1958). Reduced viability, so-called “inbreeding depression”, in bottlenecked populations is a well documented phenomenon (Frankham 1995, 2005). The relationships between reduced population size, genetic variability and inbreeding depression are not straightforward because there is a large stochastic component involved and selection coefficients may vary greatly depending on environmental conditions (Bouzat 2010). There is, however, a general trend. Because different genotypes display varying fitnesses under environmental stress (e.g., Nevo 2001) genetically diverse populations may better adapt to environmental changes. Loss of genetic diversity generally reduces this adaptive potential and increases the extinction risk of populations. The extinction risk, therefore, appears to increase under enhanced environmental stress (Bijlsma et al. 2000; Reed et al. 2002).
Lichens are highly specialized symbioses between heterotrophic fungi (the mycobiont) and autotrophic green algae and/or cyanobacteria (the photobiont). Most lichens are poor competitors, and in boreal, temperate and tropical ecosystems their contribution to biological diversity is relatively small. In polar and alpine regions, however, lichens may dominate the terrestrial vegetation and form the major part of biomass and biodiversity. In terrestrial ecosystems of the Antarctic the case is more extreme; more than 400 lichen species have been identified, while only two indigenous vascular plants and fewer than 150 bryophyte species have been found (Øvstedal & Lewis Smith 2001). The species diversity of Antarctic bryophytes and lichens decreases with increasing latitude (Peat et al. 2007), which indicates an ecological influence on species diversity. Their poikilohydric nature may explain the ability of lichens to cope with adverse environmental conditions and hence their predominance in Antarctic terrestrial habitats. Many ecophysiological studies have demonstrated the extraordinary tolerance of Antarctic lichens to drought, prolonged periods of darkness and low temperatures (Kappen 1993, 2000; Schlensog et al. 2004; Pannewitz et al. 2006).
The gross differences in species numbers between lichens, bryophytes and vascular plants may also reflect fundamentally different colonization histories. At present, virtually nothing is known about the population history of Antarctic lichens. Romeike et al. (2002) suggested that extant species’ populations represent relicts from warmer climatic periods, or result from more recent colonization events, in the shape of rare or even multiple colonization events by well adapted ecotypes (possibly followed by speciation). Although the hypothesis of Pleistocene glacial refugia in the Antarctic was recently supported by glaciological evidence and population genetic data from various organismal groups (e.g., Convey et al. 2009; De Wever et al. 2009; McGaughran et al. 2010), most invertebrate animals seem to have invaded the maritime Antarctic after the Late Glacial Maximum (Convey et al. 2008; Pugh & Convey 2008). The low degree of endemism in Antarctic lichens and bryophytes (Hertel 1988; Sancho et al. 1999; Skotnicki et al. 2000) may also be interpreted as evidence against the glacial survival of many species. Only 21% of the lichen taxa reported from the maritime Antarctic (where the majority of the species occurs) are endemic, while 55% are more or less globally distributed (Sancho et al. 1999). According to Muñoz et al. (2004), the distribution patterns of bryophytes and lichens on sub-Antarctic islands are correlated with the prevailing wind patterns, which indicates directional long distance colonization. Their results are based on species lists for different islands and therefore do not provide any information on genetic exchange between populations of the same species. The only studies that have so far addressed the question of genetic diversity in Antarctic lichen mycobionts and their photobionts were those of Romeike et al. (2002), who reported five different Trebouxia photobionts from four species of Umbilicaria collected along a transect from the continental to the maritime Antarctic, and between one and three internal transcribed spacer (ITS) haplotypes for the mycobiont of each species. Wirtz et al. (2003) found two genetic lineages of cyanobacterial photobionts that were shared by several lichen species within the same locality. Lindblom & Søchting (2008) detected 10 haplotypes of the bipolar lichen Xanthomendoza borealis. But the data set comprises sequences from both hemispheres and the genetic variability of Antarctic populations is not explicitly mentioned.
We compare here the genetic diversity of Antarctic populations of the widespread lichen Cetraria aculeata with that of populations from other continents. We use DNA sequences of six gene loci from both symbionts of the lichen as estimators for the genetic diversity in Antarctic and extra-Antarctic populations. Using different loci is important because genetic variability can vary greatly not only among species but also among different parts of a genome. In order to separate the effects of geographical isolation from those of unfavourable environmental conditions on the genetic diversity we included several populations from Arctic, Antarctic, temperate and antiboreal regions in this study. The study serves as a first step towards estimating the general genetic variability and adaptive potential of Antarctic lichen species.
Materials and methods
A total of 222 thalli of Cetraria aculeata, between 16 and 21 specimens from each of 12 populations (Table 1), were investigated in this study. Each sampling site was ca. 2500 m2 in size. Single thalli were sampled at a distance of at least 0.5 m from each other, air dried and either stored at room temperature or at −20 °C before DNA isolation. Thallus fragments were carefully checked for fungal infections and DNA was extracted from suitable lobes using the DNeasy™ Plant Mini Kit (Qiagen, Venlo, The Netherlands) following the manufacturer's protocol.
Table 1 Names, individuals per population and collection data of Cetraria aculeata samples used in this study.
| Population |
n |
Latitude / Longitude |
Locality, year and collector |
| Svalbard 1 |
17 |
78°12'34′′ N, 15°35′31′′ E |
Svalbard, Longyearbyen, 2008, S. Domaschke |
| Svalbard 4 |
20 |
78°10′45′′ N, 16°18′24′′ E |
Svalbard, Adventdalen, 2008, S. Domaschke |
| Iceland 1 |
18 |
65°52′55′′ N, 18°03′03′′ W |
Iceland, Suður-Þingeyjarsýsla, 2008, S. Domaschke & I. Ottich |
| Iceland 8 |
17 |
65°31′47′′ N, 19°31′03′′ W |
Iceland, Skagafjarðarsýsla. 2008. S. Domaschke & I. Ottich |
| Spain |
21 |
42°14′38′′ N, 06°00′33′′ W |
Spain, Castilla y León, Provincia de León, Herreros de Jamuz, 2007, S. Pérez-Ortega |
| Turkey |
20 |
40°26′49′′ N, 31°45′03′′ W |
Turkey, Bolu Province, 2007, T. Spribille & P. Lembcke |
| Kazakhstan |
16 |
53°16′44′′ N, 69°20′20′′ W |
Kazakhstan, Kokchetav area, 2007, V. Wagner |
| Chile |
19 |
52°10′08′′ S, 69°47′27′′ W |
Chile, XII region de Magalanes y de la Antartica Chilena, Punta Delgada, 2008, S. Pérez-Ortega & M. Vivas |
| Falkland |
18 |
51°41′53′′ S, 57°49′13′′ W |
Falkland Islands, East Falkland, East of Stanley, 2007, I. Ottich & C. Printzen. |
| Antarctica 1 |
17 |
62°14′47′′ S, 58°40′39′′ W |
South Shetland Islands, King George Island, 2007, I. Ottich & C. Printzen. |
| Antarctica 2 |
19 |
62°14′22′′ S, 58°39′58′′ W |
South Shetland Islands, King George Island, 2007, I. Ottich & P. Jordan |
| Antarctica 3 |
20 |
62°14′47′′ S, 58°40′07′′ W |
South Shetland Islands, King George Island, 2007, I. Ottich & P. Jordan |
Initial tests were performed to identify gene loci that simultaneously display infraspecific variability and reliably amplify in polymerase chain reactions (PCR). In the mycobiont the two anonymous loci MCM7 and TSR1 (primers in Schmitt et al. (2009)) did not amplify. Elongation factor 1 alpha (EF1; primers in Gerardo (2004)) and RNA polymerase II subunit 1 (RPB1; primers in Matheny et al. (2002)) amplified, but showed no (EF1) or very little (RPB1) variation. In the photobiont, two parts of the rps11–rpl2 gene cluster (primers in Provan et al. (2004)) did not amplify and the large chain of the ribulose bisphosphate carboxylase (rbcL; primers in Nyati (2006)) proved to be invariable. Sequencing effort was finally concentrated on the mycobiont and photobiont ITS region of the nuclear ribosomal DNA because this marker proved to be variable and was reliably amplified in the preliminary tests, and we attempted to sequence at least 20 individuals from each population. In order to verify these results, we sequenced four additional genes. For the mycobiont we sequenced part of the glyceraldehyde-3-phosphate dehydrogenase (GPD) and part of the large subunit of the mitochondrial ribosomal DNA (mtLSU). For the photobiont we sequenced part of the actin gene and part of the subunit 2 of the mitochondrial cytochrome c oxidase (COX2). Depending on the success of PCR reactions, we generated between 10 and 15 sequences per population for these gene loci.
Twenty-five µl PCR reactions containing PuReTaq Ready-to-Go PCR-Beads © (GE Healthcare, Waukesha, WI, USA), 5 µl of DNA extract and 1 µl of each of the 5′- and 3′- primers (Table 2) were set up. Cycling conditions for the mycobiont and photobiont ITS were as follows: initial denaturation of 94 °C (5′), five cycles of 94 °C (30′′), 54 °C (30′′), 72 °C (1′), 33 cycles of 94 °C (30′′), 48° C (30′′), 72 °C (1′) and final extension of 72 °C (10′) These were the cycling conditions for the GPD: initial denaturation of 95 °C (5′), 30 cycles of 95 °C (1′), 56 °C (1′), 72 °C (1′) and final extension of 72 °C (9′). These were the cycling conditions for the mtLSU: initial denaturation of 95 °C (5′), six cycles of 95 °C (30′′), 63 °C (30′′) with a reduction of 1° per cycle, 72 °C (1′), 38 cycles of 95 °C (30′′), 58 °C (30′′), 72 °C (1′) and final extension of 72 °C (10′). Cycling conditions for actin were as follows initial denaturation of 94 °C (5′), 10 cycles of 94 °C (1′), 62 °C (1′) with a reduction of 0, 5° per cycle, 72 °C (1′), 35 cycles of 94 °C (1′), 57 °C (1′), 72 °C (1′) and final extension of 72 °C (7′). These were the cycling conditions for the COX2: initial denaturation of 94 °C (5′), 14 cycles of 94 °C (30′′), 65 °C (30′′) with a reduction of 1° per cycle, 72 °C (1′), 33 cycles of 94 °C (30′′), 52 °C (30′′), 72 °C (1′) and final extension of 72 °C (10′).
Table 2 Primers used in this study.
| Primer |
Primer sequence |
Published in |
| ITS1F |
5′-CTT GTT CAT TTA GAG GAA GTA A-3′ |
Gardes & Bruns (1993) |
| ITS4 |
5′-TCC TCC GCT TAT TGA TAT GC-3′ |
|
| GPD_LM1 |
5′-CCC ACT CGT TGT CGT ACC A-3′ |
Myllys et al. (2002) |
| GPD_LM2 |
5′-ATT GGC CGC ATC GTC TTC CGC AA-3′ |
|
| ML3.A |
5′-GCT GGT TTT CTG CGA AAC CTA TAT AAG-3′ |
Printzen (2002) |
| ML4.A |
5′-GTT AGT TTG CCG AGT TCC TTA ATG-3′ |
|
| ITS1T |
5′-GGA AGG ATC ATT GAA TCT ATC GT-3′ |
Kroken & Taylor (2000) |
| ITS4T |
5′-GGT TCG CTC GCC GCT ACT A-3′ |
|
| ACT1T |
5′-CAC ACR GTR CCC ATC TAY GAG G-3′ |
Kroken & Taylor (2000) |
| ACT4T |
5′-GTT GAA CAG CAC CTC AGG GCA-3′ |
|
| COX2P2fw |
5′-GGC ATG AAA GCA TGG TTA GC-3′ |
Fernández-Mendoza et al. (2011) |
| COX2P2rev |
5′-TCT GGA TGT TAG CAA GAA CTT TGT-3′ |
|
PCR products were run on agarose gels and purified using either the QIAquick Gel Extraction Kit (Qiagen) or the peqGOLD MicroSpin Gel Extraction Kit (Peqlab, Erlangen, Germany) for gel extraction. Purified DNA was labelled with either the BigDye© Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Rotkreuz Zug, Switzerland) or with the GenomeLab™ DTCS Quick Start Kit (Beckman Coulter, Brea, CA, USA) and cycle sequenced at 94 °C (30′′), and 29 cycles of 95 °C (15′′), 45 °C (15′′) and 60 °C (4′). Sequences were determined on an ABI PRISM® 3730 DNA Analyzer (Applied Biosystems) or a CEQ™ 8800 Genetic Analysis System (Beckman Coulter) using ITS4 (mycobiont ITS), ML3.A (mtLSU), GPD_LM1 (GPD), ITS1T (photobiont ITS), ACT1T (actin) and COX2P2FW (COX2) as sequencing primers (Table 2). In cases in which sequence positions were difficult to read, the complement DNA strand was sequenced with the appropriate primers listed in Table 2. One sequence of each haplotype was run through a BLAST search and finally submitted to Genbank (Supplementary Table S6).
Eight different data sets were used in this study, the six single-gene data sets and concatenated three-gene data sets for the mycobiont and photobiont, respectively. The data sets were edited and aligned using the program Geneious v4.7 (Drummond et al. 2009) with the following alignment settings: cost matrix with 65% similarity (5.0/−4.0), gap opening penalty 14 and gap extension penalty 4. Positions 398–467 of the actin data set were not reliably alignable and excluded from further analyses. We calculated haplotype diversity (eqn. 8.4 in Nei 1987), nucleotide diversity Pi (p) (eqn. 10.5 in Nei 1987) and Tajima's D (Tajima 1989) using DnaSP v5.0 (Librado & Rozas 2009). Haplotype and nucleotide diversity of the ITS markers were calculated for single populations. Because of the relatively low number of sequences per population this was not meaningful for the other four markers. In order to test whether the genetical diversities differed significantly between geographical regions, we therefore pooled populations into four groups—Arctic, northern temperate, antiboreal and Antarctic—that contained between 33 and 72 ITS sequences or 21–51 sequences for the other markers and recalculated diversity values for these groups. Haplotype and nucleotide diversity values were compared by two-sided t-tests (eqns. 8.62 & 8.65 in Zar 1999) using the Quick Calcs t-test calculator from GraphPad software (available on the internet at http://www.graphpad.com/quickcalcs/ttest1.cfm?Format=SD). In order to assess whether our limited samples (especially for the non ITS markers) capture a considerable part of the haplotypes that are present in the four regions we calculated two estimators of absolute haplotype richness using the internet-accessible Richness Estimator v2.1 Eco-Tool (Russell 2006). The Chao 1 richness estimator (Chao 1984; Colwell & Coddington 1994) was calculated for the four groups of populations: Antarctic, Arctic, antiboreal and temperate. Due to the lack of doubletons, it was not possible to calculate Chao richness estimators for single populations.
In order to reconstruct the phylogenetic relationships between individuals, haplotype networks of the mycobiont and the photobiont were calculated from the concatenated data sets with the program TCS v1.21 (Clement et al. 2000) treating gaps as fifth character state. Instead of calculating statistical support values for connections between haplotypes, TCS uses the 95% parsimony probability criterion (Templeton et al. 1992), which means that only connections with more than 95% probability of being parsimonious are displayed. The combined data sets for the photobiont and mycobiont contained only those individuals, from which all genes were successfully sequenced.
Results
A total of 951 DNA sequences was generated for this study: 200 mycobiont ITS (alignment length: 497 bp), 124 GPD (alignment length: 545 bp), 160 mtLSU (alignment length: 828 bp), 219 photobiont ITS (alignment length: 572 bp), 110 COX2 (alignment length: 475 bp) and 138 actin sequences (alignment length: 444 bp). The concatenated mycobiont data set (ITS, GPD and mtLSU) comprises 99 sequences and has a total length of 1870 sites, the corresponding photobiont data set (ITS, Actin and COX2) consists of 91 sequences and is 1491 bp long. The calculation of Tajima's D (Supplementary Table S7) for the six markers used in this study shows some signal of balancing selection only for GPD. For all other markers the hypothesis of neutrality can not be rejected.
Indices of genetic diversity are summarized separately for the two symbionts in Tables 3 and 4. The genetic diversity of both symbionts of Cetraria aculeata varies greatly among populations and geographical regions and the observed diversity patterns of the mycobiont and the photobiont differ markedly. For all three gene loci of the mycobiont, the haplotype diversity is lowest in Antarctica (Table 3). This is most conspicuous for mtLSU, in which all 51 sequences belong to the same haplotype. The genetic diversity of the mycobiont is by far the highest in Arctic populations, while temperate and antiboreal populations occupy an intermediate position. Antiboreal populations have a higher GPD and mtLSU haplotype diversity, while temperate populations are slightly more variable at the ITS locus. The nucleotide diversity shows an overall similar pattern, but temperate populations are more variable at the GPD and mtLSU locus than antiboreal ones. The observed and estimated numbers of haplotypes vary also greatly among the four geographical regions. We found between one and four haplotypes of each marker in Antarctic and antiboreal populations, which covers the expected total number of haplotypes in these regions. In the temperate and Arctic population groups the number of observed haplotypes ranges between three and 17 and the expected total number can be even higher, reaching values of up to 22 for ITS. The two-sided t-test shows that the differences in haplotype diversity among most geographical groups are highly significant (Tables 3, 4; see Supplementary Table S8 for comparisons of single populations based on the ITS marker). The only non-significant comparisons were between the temperate and the antiboreal group at the ITS locus.
Table 3 Number of individuals (N), number of haplotypes (H), estimated absolute number of haplotypes following Chao 1 and 2 (S1/2), number of haplotypes per single population (H/pop.), haplotype diversity (h) and nucleotide diversity (Pi) of the mycobiont ITS, GPD and mtLSU sequences. Comparison of diversity indices for per locus haplotype diversity (below each blank diagonal) and nucleotide diversities (above each blank diagonal) of different population groups of Cetraria aculeata and results from the two-sided t-test (significant differences after Bonferroni correction in boldface). Values in boldface for S1 and S2 indicate >50% deviation between observed and expected numbers of haplotypes.
| Region |
Gene |
N |
H |
S1 |
S2 |
H/pop. |
h |
Pi |
Arctic |
Temperate |
Antiboreal |
Antarctic |
| Arctic |
ITS |
68 |
17 |
18.3 |
21.8 |
4–8 |
0.909 |
0.01432 |
|
<0.0001
|
<0.0001
|
<0.0001
|
| Temperate |
|
47 |
7
|
17
|
12
|
1–5 |
0.566 |
0.00130 |
<0.0001
|
|
0.1210 |
<0.0001
|
| Antiboreal |
|
33 |
4 |
4 |
4.2 |
3 |
0.532 |
0.00167 |
<0.0001
|
0.0262 |
|
<0.0001
|
| Antarctic |
|
52 |
2 |
2 |
2 |
1–2 |
0.038 |
0.00008 |
<0.0001
|
<0.0001
|
<0.0001
|
|
| Arctic |
GPD |
43 |
6 |
7 |
6.2 |
3–5 |
0.722 |
0.01342 |
|
<0.0001
|
<0.0001
|
<0.0001
|
| Temperate |
|
36 |
3 |
4 |
3.7 |
2–3 |
0.160 |
0.00317 |
<0.0001
|
|
<0.0001
|
<0.0001
|
| Antiboreal |
|
24 |
2 |
2 |
2 |
2 |
0.228 |
0.00042 |
<0.0001
|
0.0055
|
|
<0.0001
|
| Antarctic |
|
21 |
2 |
2 |
2 |
1–2 |
0.095 |
0.00017 |
<0.0001
|
0.0053
|
<0.0001
|
|
| Arctic |
mtLSU |
46 |
6 |
7
|
13.5
|
2–3 |
0.609 |
0.00077 |
|
<0.0001
|
0.0061
|
<0.0001
|
| Temperate |
|
43 |
5 |
12
|
9.3
|
1–5 |
0.179 |
0.00128 |
<0.0001
|
|
0.0686 |
<0.0001
|
| Antiboreal |
|
20 |
2 |
2 |
2 |
2 |
0.395 |
0.00096 |
<0.0001
|
<0.0001
|
|
<0.0001
|
| Antarctic |
|
51 |
1 |
1 |
1 |
1 |
0 |
0 |
<0.0001
|
<0.0001
|
<0.0001
|
|
Table 4 Number of individuals (N), number of haplotypes (H), estimated absolute number of haplotypes following Chao 1 and 2 (S1/2), number of haplotypes per single population (H/pop.), haplotype diversity (h) and nucleotide diversity (Pi) of the photobiont ITS, COX2 and Actin sequences. Comparison of diversity indices for per locus haplotype diversity (below each blank diagonal) and nucleotide diversities (above each blank diagonal) of different population groups of Trebouxia jamesii and results from the two-sided t-test (significant differences after Bonferroni correction in boldface). Values in boldface for H, S1 and S2 indicate >50% deviation between observed and expected numbers of haplotypes.
| Region |
Gene |
N |
H |
S1 |
S2 |
H/pop. |
h |
Pi |
Arctic |
Temperate |
Antiboreal |
Antarctic |
| Arctic |
ITS |
71 |
14 |
32
|
38.8
|
2–8 |
0.632 |
0.01538 |
|
<0.0001
|
<0.0001
|
<0.0001
|
| Temperate |
|
55 |
12 |
19.5
|
22
|
3–7 |
0.820 |
0.02233 |
<0.0001
|
|
<0.0001
|
<0.0001
|
| Antiboreal |
|
37 |
11 |
21.5
|
17
|
6–7 |
0.799 |
0.00684 |
<0.0001
|
0.0070
|
|
<0.0001
|
| Antarctic |
|
56 |
3 |
3 |
3 |
2–3 |
0.455 |
0.00082 |
<0.0001
|
<0.0001
|
<0.0001
|
|
| Arctic |
COX2 |
38 |
4 |
4 |
4 |
1–4 |
0.670 |
0.00295 |
|
<0.0001
|
<0.0001
|
<0.0001
|
| Temperate |
|
30 |
3 |
4 |
4.2 |
1–3 |
0.618 |
0.00486 |
<0.0001
|
|
<0.0001
|
<0.0001
|
| Antiboreal |
|
21 |
3 |
3 |
4.5 |
1–2 |
0.571 |
0.00130 |
<0.0001
|
0.0021
|
|
<0.0001
|
| Antarctic |
|
21 |
1 |
1 |
1 |
1 |
0 |
0 |
<0.0001
|
<0.0001
|
<0.0001
|
|
| Arctic |
Actin |
25 |
9 |
11 |
10.9 |
2–4 |
0.753 |
0.01237 |
|
<0.0001
|
0.8969 |
<0.0001
|
| Temperate |
|
45 |
13 |
23.5
|
25.2
|
1–8 |
0.817 |
0.07633 |
<0.0001
|
|
<0.0001
|
<0.0001
|
| Antiboreal |
|
24 |
6 |
12
|
8.5 |
3–4 |
0.703 |
0.01270 |
0.0204 |
<0.0001
|
|
<0.0001
|
| Antarctic |
|
44 |
2 |
2 |
2 |
1–2 |
0.474 |
0.00332 |
<0.0001
|
<0.0001
|
<0.0001
|
|
All sequenced photobionts belong to Trebouxia jamesii (Fernández-Mendoza et al. 2011). Antarctic populations display the smallest observed and expected numbers of haplotypes and the lowest values for haplotype and nucleotide diversity at all three loci (Table 4). The nucleotide diversity is consistently highest in the temperate group, but the Arctic group shows a slightly higher haplotype diversity at the COX2 locus. The Chao 1 and 2 richness estimators again indicate that the Antarctic populations were sampled comprehensively, while our sample is not comprehensive in the other regions, except for the COX2 locus. The only non-significant difference in haplotype diversity is observed between Arctic and antiboreal populations at the actin locus.
Comparisons between the geographical regions are biased by the fact that the geographical distances between populations vary greatly among the groups. Due to logistic constraints, all three Antarctic populations were sampled on King George Island, while the populations from Spain and Kazakhstan in the temperate group are more than 5000 km apart from each other. Table 5 shows haplotype numbers and diversity values at the ITS locus for each single mycobiont and photobiont populations. It is evident that the Antarctic mycobiont populations are genetically more uniform than most of the other populations. The only exceptions are the populations from Turkey and Kazakhstan, which comprise only one and two haplotypes, respectively, and consequently have values of haplotype and nucleotide diversity comparable to that of the Antarctic populations. When the three closely spaced Antarctic populations are pooled, their diversity is lower than that of any other single population in the data set except Turkey. Due to the limited sampling of GPD and mtLSU genes, diversity indices were not calculated for these markers. Nevertheless, a comparison of the numbers of haplotypes found in single populations (Table 3) shows lower haplotype numbers in Antarctic populations, although twice as many individuals were sampled here. The same pattern is obvious for the photobiont (Tables 4, 5). Although the differences in genetic diversity are less pronounced, the number of observed and expected haplotypes and the nucleotide diversity are considerably lower in Antarctic populations. Only one population from Iceland and the population from Kazakhstan have comparably low diversity levels.
Table 5 Number of haplotypes (H), haplotype diversity (h) and nucleotide diversity (Pi) in photobiont and mycobiont ITS of Cetraria aculteata in 12 different populations and four geographical regions (boldface).
| |
Mycobiont |
Photobiont |
| |
H |
h |
Pi |
H |
h |
Pi |
| Arctic |
17 |
0.909 |
0.01432 |
14 |
0.632 |
0.01538 |
| Iceland 1 |
6 |
0.772 |
0.01363 |
4 |
0.331 |
0.00286 |
| Iceland 8 |
5 |
0.825 |
0.01456 |
2 |
0.118 |
0.00103 |
| Svalbard 1 |
4 |
0.699 |
0.01124 |
4 |
0.676 |
0.01914 |
| Svalbard 4 |
8 |
0.846 |
0.01364 |
8 |
0.795 |
0.01946 |
| Temperate |
7 |
0.566 |
0.00130 |
12 |
0.820 |
0.02233 |
| Kazakhstan |
2 |
0.133 |
0.00054 |
3 |
0.242 |
0.00044 |
| Spain |
5 |
0.405 |
0.00091 |
7 |
0.763 |
0.01996 |
| Turkey |
1 |
0 |
0 |
4 |
0.585 |
0.01089 |
| Antiboreal |
4 |
0.532 |
0.00167 |
11 |
0.799 |
0.00684 |
| Chile |
3 |
0.569 |
0.00214 |
6 |
0.538 |
0.00738 |
| Falkland |
3 |
0.514 |
0.00113 |
7 |
0.725 |
0.00177 |
| Antarctic |
2 |
0.038 |
0.00008 |
3 |
0.455 |
0.00082 |
| Antarctica 1 |
2 |
0.133 |
0.00027 |
2 |
0.485 |
0.00085 |
| Antarctica 2 |
1 |
0 |
0 |
2 |
0.118 |
0.00018 |
| Antarctica 3 |
1 |
0 |
0 |
3 |
0.574 |
0.00110 |
The haplotype network of the mycobiont (Fig. 1a) based on the concatenated ITS, GPD and mtLSU data set shows almost no overlap in haplotype composition between regions. Haplotypes from Antarctica, the Falkland Islands and Chile form a monophyletic group. Only one of the haplotypes within this group is shared among Antarctic and antiboreal populations. Another well-delimited, monophyletic group comprises all haplotypes from Turkey, Spain and Kazakhstan. In between these two clades there are five groups of Arctic haplotypes that are separated by between eight and 21 mutational steps.
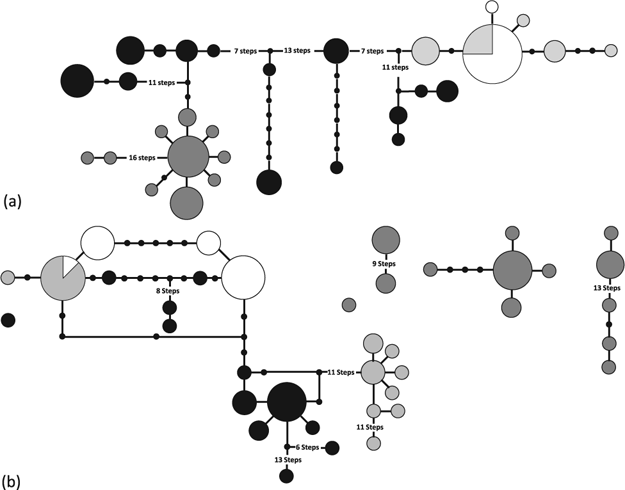
Fig. 1
Haplotype networks for (a) the combined mycobiont data set of ITS, GPD and mtLSU and (b) the combined photobiont data set of ITS, Actin and COX2. Circles represent haplotypes. The size of each circle is proportional to the number of individuals sharing this haplotype. Lines between circles represent mutational steps. Only those connections are displayed that have a probability of at least 95% of being parsimonious. Colours indicate the geographical origin of the individuals as follows: black—Arctic, dark grey—temperate, light grey—antiboreal and white—Antarctic.
The haplotype network of the photobiont based on the concatenated ITS, Actin and COX2 data set (Fig. 1b) resembles the mycobiont network in that only one haplotype is shared among Antarctic and antiboreal populations. The rest of the haplotypes is restricted to a single geographical region. However, the genetic diversity is considerably larger. As a result, the network falls into four unconnected subnetworks and two single haplotypes. The largest subnetwork connects all but one of the Arctic haplotypes with those from the Southern Hemisphere. Three loops in the network indicate homoplasy, mostly within the Actin locus. Haplotypes from the antiboreal populations form two distantly related clades on this network. It remains unclear whether the Antarctic haplotypes are monophyletic. None of the haplotypes from Spain, Turkey and Kazakhstan is connected to the main network. They appear as three unconnected clades and a single isolated haplotype.
Discussion
Population structure and phylogeography
Of the studies that have investigated the genetic diversity of lichens at a population level (Zoller et al. 1999; Kroken & Taylor 2000; Tibell 2001; Printzen & Ekman 2002; Walser et al. 2003; Arnerup et al. 2004; Palice & Printzen 2004; Piercey-Normore 2004, 2006; Walser et al. 2005; Lindblom & Ekman 2006, 2007; Ohmura et al. 2006; Kotelko et al. 2008; Baloch & Grube 2009; Mattsson et al. 2009; Geml et al. 2010), most were not concerned with comparisons of population-level diversities over larger geographical ranges. This is at least partly due to the fact that the distributional ranges of most lichens are very large, which makes it difficult if not impossible to sample them comprehensively. In this study, we did not attempt to compare samples from the entire range of the species, but instead concentrated on the geographical and climatical extremes of the range and ecological niche of Cetraria aculeata. Our data show that the genetical diversity of both symbionts of C. aculeata is geographically unevenly distributed. This result is not surprising, but confirms previous studies on widely disjunct lichens. Hotspots of genetical diversity were, for example, detected in western North America for Cavernularia hultenii (Printzen et al. 2003) and Lobaria pulmonaria (Walser et al. 2005), in Morocco and the Caucasus for Letharia vulpina (Arnerup et al. 2004) and in the Ecuadorian Andes for Trapeliopsis glaucolepidea (Palice & Printzen 2004). In Cetraria aculeata, mycobiont populations are most diverse in the Arctic, while photobionts are most diverse in the temperate zone. In contrast to the abovementioned studies, we also detected a region with strongly reduced diversity in the Antarctic. Whether reduced diversity in the Antarctic is a general trend in lichens is at present impossible to say because comparative studies of Antarctic and bipolar lichens are so far missing (Printzen 2008). Romeike et al. (2002) detected three haplotypes in 11 samples of U. antarctica sampled along a transect in the western Antarctic. However, their results are based on a very small sample. Wirtz et al. (2008), on the other hand, found exactly the opposite pattern, relatively high levels of diversity in Antarctic and Patagonian populations of Usnea lambii and reduced diversity in western North America. This difference probably reflects the different evolutionary history of U. lambii and C. aculeata. Usnea lambii belongs to the Neuropogon group of Usnea, which is almost entirely confined to the Southern Hemisphere. The reduced genetic diversity in western North America is probably due to founder effects during long distance dispersal to the Northern Hemisphere. The reduced genetic variability of Antarctic populations of C. aculeata could be the result of a similar effect: long distance colonization of the Antarctic from northern populations and an associated population size bottleneck. A pattern of reduced genetical diversity in leading edge populations has frequently been observed in species that expanded their ranges after the last ice age (Hewitt 2004). The haplotype networks in Fig. 1 confirm that individuals from the Southern Hemisphere are more closely related to Arctic than to temperate haplotypes, and that only one lineage of closely related haplotypes is present in the Antarctic populations that were sampled by us. The most likely scenario would be a range expansion along the Andes, perhaps in connection with glacial cycles. Diaspore exchange between the hemispheres was discussed by Galloway & Aptroot (1995), who point out that many bipolar lichen species have populations at high altitudes along major mountain ranges between the polar regions. This is also the case for Cetraria aculeata, which occurs, for example, in the high Andes of Bolivia and Peru.
The influence of selection on genetic diversity
Selection is another factor that may reduce the genetic variability of populations (Bulmer 1976). Although one could imagine that the rigorous environmental conditions of Antarctic habitats select strongly against poorly adapted genotypes, this does not seem to be the reason for the small genetic diversity observed in populations from King George Island. The environmental conditions are hardly less favourable in Svalbard, yet the population Svalbard 4 displays the highest genetic diversity—for both symbionts—of any population included in this study (Table 5). The other Arctic populations are also more variable than the pooled Antarctic populations. Moreover, values for Tajima's D indicate that most of the markers used in this study are selectively neutral. The only exception is GPD which, however, showed a signal of balancing selection and not positive selection as expected under increased environmental stress.
A recent report of increased genetic diversity due to selection under harsh environmental conditions (Kaeuffer et al. 2006) indicates that the relationship between selection and genetic diversity is complex. Kaeuffer et al. (2006) measured heterozygosity in diploid mouflons on Kerguelen Island and explained the increase in heterozygosity with strong selection against homozygotes. Cetraria aculeata is, however, a haploid species with mostly asexual, clonal reproduction (we did not observe a single individual with apothecia in the Antarctic populations sampled by us). Consequently, selection can be expected to affect genetic variability more directly, even at presumably neutral loci, because most of the genes are effectively linked.
High diversity of Arctic mycobiont populations
It is at present impossible to conclusively explain the high variability of Arctic mycobiont populations. The haplotype networks (Fig. 1) as well as diversity indices show that the genetic differences between the five Arctic lineages are more pronounced than the differences between the more widely spaced temperate populations. This pattern could be explained by population fragmentation during the Pleistocene. Subsequent admixture of genetically divergent lineages in Iceland and Svalbard could then account for the high genetic diversity of these populations. Comparatively high levels of genetic diversity in Svalbard are also reported from other species (e.g., Abbott et al. 2000; Marthinsen et al. 2008). Alsos et al. (2007) reported the postglacial colonization of Svalbard by plants from several source populations and indicate ongoing long-distance dispersal between different Arctic regions. Further evidence is provided by Geml et al. (2010), who demonstrated postglacial long distance dispersal between North America and Europe for the lichens Flavocetraria cucullata and F. nivalis. The propagation mode and the Northern Hemispheric distribution patterns of these two species resemble that of Cetraria aculeata. Long distance dispersal from different glacial refugia and admixture from different source populations could thus explain the high diversity of Arctic populations. However, we are unable to test this at present because of our limited sampling in the Arctic.
Symbiont associations
In contrast to the mycobiont, photobiont populations of Trebouxia jamesii were most diverse in the temperate region. At first glance it is counterintuitive that a largely sterile lichen-forming fungus as C. aculeata should be able to associate with such a wide variety of photobiont lineages. However, many studies have shown that even entirely sterile lichens can frequently switch photobionts (Piercey-Normore & DePriest 2001; Ohmura et al. 2006; Robertson & Piercey-Normore 2007; Nelsen & Gargas 2009). The differences in genetic variability between Arctic and temperate populations are relatively small compared to the differences observed in the mycobiont and there is at present no evidence for a relationship between climate and the genetic diversity of photobionts. Nevertheless, climate influences with which photobiont lineage C. aculeata associates in a certain region (Fig. 1b; Fernández-Mendoza et al. 2011). This is in line with the results of previous studies on photobiont association in lichens. Muggia et al. (2008) found that alpine lineages of the Tephromela atra group associate with a different lineage of Trebouxia simplex than Mediterranean samples. A similar difference between alpine and Mediterranean photobionts was detected in Lecanora rupicola (Blaha et al. 2006). The mycobiont of the epiphytic western North American endemic Ramalina menziesii associates with different photobiont lineages depending on the phorophyte (Werth & Sork 2010). The fact that photobiont diversity decreases significantly towards the Antarctic but less pronouncedly towards the Arctic suggests that ecological factors play a minor role in determining the diversity of Antarctic photobiont populations. As for the mycobiont demographic history, founder effects in connection with long distance dispersal offers the best explanation for the observed pattern.
The influence of sampling bias
Finally, the reduced genetic variability of Antarctic populations could be an artefact caused by unequal geographical sampling. The three Antarctic populations included in this study were all sampled on King George Island. The antiboreal, temperate and Arctic populations are separated by much larger distances of up to a thousand kilometres. The question is therefore whether we underestimated the haplotype diversity in the Antarctic populations or overestimated the diversity in the other regions by pooling data from geographically distant populations and whether a sample of 20 thalli is enough to adequately reflect the true haplotype diversity of a population. While “stopping rules”—rules that indicate the point beyond which no more sampling is necessary—can be defined for the inference of species diversity (Magurran 2004) this is impossible for measuring genetic diversity. Lindblom (2009) therefore suggested using richness estimators to test a posteriori whether the sampling revealed all haplotypes potentially present. The Chao estimators of haplotype (species) richness (Tables 3, 4) indicate that the Antarctic populations were sampled comprehensively. The expected number equals the observed number of haplotypes in the three pooled populations. It is reasonable to assume that the inferred haplotype and nucleotide diversities are also near the true parameter values. Table 5 shows that, at least for the ITS locus, these values are lower for the pooled Antarctic populations than for any other single population except Turkey. At present, it remains uncertain whether the other four loci display the same pattern. Because there were only around 10 sequences available for each single population we did not calculate diversity indices for these loci and single populations.
Conclusions
Whether the low genetic diversity of Antarctic mycobiont and photobiont populations of Cetraria aculeata will impede the adaptation of this species to increasing temperatures depends on the ecophysiological capacities of the few Antarctic genotypes. At present, nothing is known about this aspect. Even if Antarctic individuals were unable to compete with invading species it is unlikely that C. aculeata as a species will disappear from the Antarctic because it is common in Patagonia and ecotypes adapted to warmer climatic conditions might extend their ranges from there. There is, however, a certain danger that the Antarctic mycobiont and photobiont genotypes observed by us may not survive under a scenario of global warming.
Acknowledgements
The staff of the Grunelius-Möllgaard Laboratory for Molecular Evolution, especially Heike Kappes, and Selina Becker, Jasmin Seifried (Frankfurt) and Bastian Millgramm (Kassel) are thanked for technical support in the laboratory, Indra Ottich and Patrick Jordan (Frankfurt) for field assistance and Toby Spribille (Graz), Viktoria Wagner (Halle) and Sergio Pérez-Ortega (Madrid) for population samples of C. aculeata. Funding by the German Research Foundation grant no. Pr 567/12-1, the European Union through Synthesys grant no. ES-TAF 3621 to CP and the Marga and Kurt Möllgaard Foundation are gratefully acknowledged. The present study was financially supported by the research funding programme LOEWE–Landes-Offensive zur Entwicklung Wissenschaftlich-ökonomischer Exzellenz of Hesse's Ministry of Higher Education, Research, and the Arts.
References
Abbott
R.J.,
Smith
L.C.,
Milne
R.I.,
Crawford
R.M.M.,
Wolff
K.
&
Balfour
J. 2000.
Molecular analysis of plant migration and refugia in the Arctic. Science 289,
1343–1346.
[Crossref]
Alsos
I.G.,
Bronken Eidesen
P.,
Ehrich
D.,
Skrede
I.,
Westergaard
K.,
Jacobsen
G.H.,
Landvik
J.Y.,
Taberlet
P.
&
Brochmann
C. 2007.
Frequent long-distance plant colonization in the changing Arctic. Science 316,
1606–1609.
[Crossref]
Arnerup
J.,
Högberg
N.
&
Thor
G. 2004.
Phylogenetic analysis of multiple loci reveal the population structure within Letharia in the Caucasus and Morocco. Mycological Research 108,
311–316.
[Crossref]
Baloch
E.
&
Grube
M. 2009.
Pronounced genetic diversity in tropical epiphyllous lichen fungi. Molecular Ecology 18,
2185–2197.
[Crossref]
Bijlsma
R.,
Bundgaard
J.
&
Boerema
A.C. 2000.
Does inbreeding affect the extinction risk of small populations? Predictions from Drosophila. Journal of Evolutionary Biology 13,
502–514.
[Crossref]
Blaha
J.,
Baloch
E.
&
Grube
M. 2006.
High photobiont diversity associated with the euryoecious lichen-forming ascomycete Lecanora rupicola (Lecanoraceae, Ascomycota). Biological Journal of the Linnean Society 88,
283–293.
[Crossref]
Bouzat
J.L. 2010.
Conservation genetics of population bottlenecks: the role of chance, selection, and history. Conservation Genetics 11,
463–478.
[Crossref]
Bulmer
M.G. 1976.
The effect of selection on genetic variability: a simulation study. Genetical Research 28,
101–117.
[Crossref]
Chao
A. 1984.
Nonparametric estimation of the number of classes in a population. Scandinavian Journal of Statistics 11,
265–270.
Clement
M.,
Posada
D.
&
Crandall
K. 2000.
TCS: a computer program to estimate gene genealogies. Molecular Ecology 9,
1657–1660.
[Crossref]
Colwell
R.K.
&
Coddington
J.A. 1994.
Estimating terrestrial biodiversity through extrapolation. Philosophical Transactions of the Royal Society of London B 345,
101–118.
[Crossref]
Convey
P.,
Gibson
J.A.E.,
Hillenbrand
C.D.,
Hodgson
D.A.,
Pugh
P.J.A.,
Smellie
J.L.
&
Stevens
M.I. 2008.
Antarctic terrestrial life—challenging the history of the frozen continent? Biological Reviews 83,
103–117.
[Crossref]
Convey
P.,
Stevens
M.I.,
Hodgson
D.A.,
Smellie
J.L.,
Hillenbrand
C.D.,
Barnes
D.K.A.,
Clarke
A.,
Pugh
P.J.A.,
Linse
K.
&
Cary
C. 2009.
Exploring biological constraints on the glacial history of Antarctica. Quaternary Science Reviews 28,
3035–3048.
[Crossref]
Cornelissen
J.H.C.,
Callaghan
T.V.,
Alatalo
J.M.,
Michelsen
A.,
Graglia
E.,
Hartley
A.E.,
Hik
D.S.,
Hobbie
S.E.,
Press
M.C.,
Robinson
C.H.,
Henry
G.H.R.,
Shaver
G.R.,
Phoenix
G.K.,
Gwynn Jones
D.,
Jonasson
S.,
Chapin
F.S. III,
Molau
U.,
Neill
C.,
Lee
J.A.,
Melillo
J.M.,
Sveinbjörnsson
B.
&
Aerts
R. 2001.
Global change and Arctic ecosystems: is lichen decline a function of increases in vascular plant biomass? Journal of Ecology 89,
984–994.
[Crossref]
De Wever
A.,
Leliaert
F.,
Verleyen
E.,
Vaormelingen
P.,
Van der Gucht
K.,
Hodgson
D.A.,
Sabbe
K.
&
Vyverman
W. 2009.
Hidden levels of phylodiversity in Antarctic green algae: further evidence for the existence of glacial refugia. Proceedings of the Royal Society of London B 276,
3591–3599.
[Crossref]
Drummond
A.J.,
Ashton
B.,
Cheung
M.,
Heled
J.,
Kearse
M.,
Moir
R.,
Stones-Havas
S.,
Thierer
T.
&
Wilson
A. 2009. Geneious version 4.7. Accessed on the internet at http://www.geneious.com
on 3 February 2010.
Fernández-Mendoza
F.,
Domaschke
S.,
García
M.A.,
Jordan
P.,
Martín
M.P.
&
Printzen
C. 2011.
Population structure of mycobionts and photobionts of the widespread lichen Cetraria aculeata. Molecular Ecology 20,
1208–1232.
[Crossref]
Fisher
R.A.
1958. The genetical theory of natural selection. 2nd edn. New York: Dover.
Frankham
R. 1995.
Conservation genetics. Annual Review of Genetics 29,
305–327.
[Crossref]
Frankham
R. 2005.
Genetics and extinction. Biological Conservation 126,
131–140.
[Crossref]
Galloway
D.J.
&
Aptroot
A. 1995.
Bipolar lichens: a review. Cryptogamic Botany 5,
184–191.
Gardes
M.
&
Bruns
T.D. 1993.
ITS primers with enhanced specificity for basidiomycetes—application to the identification of mycorrhizae and rusts. Molecular Ecology 2,
113–118.
[Crossref]
Geml
J.,
Kauff
F.,
Brochmann
C.
&
Taylor
D.L. 2010.
Surviving climate changes: high genetic diversity and transoceanic gene flow in two Arctic–alpine lichens, Flavocetraria cucullata and F. nivalis (Parmeliaceae, Ascomycota). Journal of Biogeography 37,
1529–1542.
Gerardo
N.M. 2004. The nature of parasite specialization in the fungus-growing ant symbiosis,. PhD thesis,
University of Texas at Austin.
Hertel
H. 1988.
Problems in monographing Antarctic crustose lichens. Polarforschung 58,
65–76.
Hewitt
G.M. 2004.
Genetic consequences of climatic oscillations in the Quaternary. Philosophical Transactions of the Royal Society London B 359,
183–195.
[Crossref]
Ingvarsson
P.K. 2001.
Restoration of genetic variation lost—the genetic rescue hypothesis. Trends in Ecology and Evolution 16,
62–63.
[Crossref]
Kaeuffer
R.,
Coltman
D.W.,
Chapuis
J.L.,
Pontier
D
&
Réale
D. 2006.
Unexpected heterozygosity in an island mouflon population founded by a single pair of individuals. Proceedings of the Royal Society London B 274,
527–533.
[Crossref]
Kappen
L. 1993.
Plant activity under snow and ice, with particular reference to lichens. Arctic 46,
297–302.
Kappen
L. 2000.
Some aspects of the great success of lichens in Antarctica. Antarctic Science 12,
314–324.
[Crossref]
Kotelko
R.,
Doering
M.
&
Piercey-Normore
M.D. 2008.
Species diversity and genetic variation of terrestrical lichens and bryophytes in a boreal jack pine forest of central Canada. Bryologist 111,
594–606.
[Crossref]
Kroken
S.
&
Taylor
J.W. 2000.
Phylogenetic species, reproductive mode, and specificity of the green alga Trebouxia forming lichens with the fungal genus Letharia. Bryologist 103,
645–660.
Librado
P.
&
Rozas
J. 2009.
DnaSP version 5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25,
1451–1452.
[Crossref]
Lindblom
L. 2009.
Sample size and haplotype richness in population samples of the lichen-forming ascomycete Xanthoria parietina. Lichenologist 41,
529–535.
[Crossref]
Lindblom
L.
&
Ekman
S. 2006.
Genetic variation and population differentiation in the lichen-forming ascomycete Xanthoria parietina on the island Storfosna, central Norway. Molecular Ecology 15,
1545–1559.
[Crossref]
Lindblom
L.
&
Ekman
S. 2007.
New evidence corroborates population differentiation in Xanthoria parietina. Lichenologist 39,
259–271.
[Crossref]
Lindblom
L.
&
Søchting
U. 2008.
Taxonomic revision of Xanthomendoza borealis and Xanthoria mawsonii (Lecanoromycetes, Ascomycota). Lichenologist 40,
399–409.
[Crossref]
Magurran
A.E.
2004. Measuring biological diversity. Oxford: Blackwell Publishing.
Marthinsen
G.,
Wennerberg
L.,
Pierce
E.P.
&
Lifjeld
J.T. 2008.
Phylogeographic origin and genetic diversity of dunlin Calidris alpina in Svalbard. Polar Biology 31,
1409–1420.
[Crossref]
Matheny
P.B.,
Liu
Y.J.,
Ammirati
J.F.
&
Hall
B.D. 2002.
Using RPB1 sequences to improve phylogenetic interference among mushrooms (Inocybe, Agaricales). American Journal of Botany 89,
688–698.
[Crossref]
Mattsson
J.-E.,
Hansson
A.-C.
&
Lindblom
L. 2009.
Genetic variation in relation to substratum preferences of Hypogymnia physodes. Lichenologist 41,
547–555.
[Crossref]
McGaughran
A.,
Torricelli
G.,
Carapelli
A.,
Frati
F.,
Stevens
M.I.,
Convey
P.
&
Hogg
I.D. 2010.
Contrasting phylogeographic patterns for springtails reflect different evoutionary histories between the Antarctic Peninsula and continental Antartica. Journal of Biogeography 37,
103–119.
[Crossref]
Muggia
L.,
Grube
M.
&
Tretiach
M. 2008.
Genetic diversity and photobiont associations in selected taxa of the Tephromela atra group (Lecanorales, lichenized Ascomycota). Mycological Progress 7,
147–160.
[Crossref]
Muñoz
J.,
Felicismo
M.,
Cabezas
F.,
Burgaz
A.R.
&
Martínez
I. 2004.
Wind as a long-distance dispersal vehicle in the Southern Hemisphere. Science 304,
1144–1147.
[Crossref]
Myllys
L.,
Stenroos
S.
&
Thell
A. 2002.
New genes for phylogenetic studies of lichenized fungi: glyceraldehyde-3-phosphate dehydrogenase and beta-tubulin genes. Lichenologist 34,
237–246.
[Crossref]
Nei
M.
1987. Molecular evolutionary genetics. New York: Columbia University Press.
Nei
M.,
Maruymana
T.
&
Chakraborthy
R. 1975.
The bottleneck effect and genetic variability in populations. Evolution 29,
1–10.
[Crossref]
Nelsen
M.P.
&
Gargas
A. 2009.
Symbiont flexibility in Thamnolia vermicularis (Pertusariales: Icmadophilaceae). Bryologist 122,
404–417.
[Crossref]
Nevo
E. 2001.
Evolution of genome-phenome diversity under environmental stress. Proceedings of the National Academy of Sciences of the United States of America 98,
6233–6240.
[Crossref]
Nyati
S. 2006. Photobiont diversity in Teloschistaceae (Lecanoromycetes). PhD thesis,
University of Zürich.
Ohmura
Y.,
Kawachi
M.,
Kasai
F.
&
Watanabe
M.M. 2006.
Genetic combinations of symbionts in the vegetatively reproducing lichen, Parmotrema tinctorum, based on ITS rDNA sequences. Bryologist 109,
43–59.
Øvstedal
D.O.
&
Lewis Smith
R.I.
2001. Lichens of Antarctica and South Georgia. A guide to their identification and ecology. Cambridge: Cambridge University Press.
Palice
Z.
&
Printzen
C. 2004.
Genetic variability in tropical and temperate populations of Trapeliopsis glaucolepidea: evidence against long-range dispersal in a lichen with disjunct distribution. Mycotaxon 90,
43–54.
Pannewitz
S.,
Green
T.G.A.,
Schlensog
M.,
Seppelt
R.,
Sancho
L.
&
Schroeter
B. 2006.
Photosynthetic performance of Xanthoria mawsonii C. W. Dodge in coastal habitats, Ross Sea region, continental Antarctica. Lichenologist 38,
67–81.
[Crossref]
Peat
H.J.,
Clarke
A.
&
Convey
P. 2007.
Diversity and biogeography of the Antarctic flora. Journal of Biogeography 34,
132–146.
[Crossref]
Piercey-Normore
M.D. 2004.
Selection of algal genotypes by three species of lichen fungi in the genus Cladonia. Canadian Journal of Botany 82,
947–961.
[Crossref]
Piercey-Normore
M.D. 2006.
The lichen-forming ascomycete Evernia mesomorpha associates with multiple genotypes of Trebouxia jamesii. New Phytologist 169,
331–344.
[Crossref]
Piercey-Normore
M.D.
&
DePriest
P.T. 2001.
Algal switching among lichen symbioses. American Journal of Botany 88,
1490–1498.
[Crossref]
Printzen
C. 2002.
Fungal specific primers for PCR-amplification of mitochondrial LSU in lichens. Molecular Ecology Notes 2,
130–132.
[Crossref]
Printzen
C. 2008.
Uncharted terrain: the phylogeography of Arctic and boreal lichens. Plant Ecology & Diversity 1,
265–271.
[Crossref]
Printzen
C.
&
Ekman
S. 2002.
Genetic variability and its geographical distribution in the widely disjunct Cavernularia hultenii. Lichenologist 34,
101–111.
[Crossref]
Printzen
C.,
Ekman
S.
&
Tønsberg
T. 2003.
Phylogeography of Cavernularia hultenii: evidence for slow genetic drift in a widely disjunct lichen. Molecular Ecology 12,
1473–1486.
[Crossref]
Provan
J.,
Murphy
S.
&
Maggs
C.A. 2004.
Universal plastid primers for Chlorophyta and Rhodophyta. European Journal of Phycology 39,
43–50.
[Crossref]
Pugh
P.J.A.
&
Convey
P. 2008.
Surviving out in the cold: Antarctic endemic invertebrates and their refugia. Journal of Biogeography 35,
2176–2186.
[Crossref]
Reed
D.H.,
Briscoe
D.A.
&
Frankham
R. 2002.
Inbreeding and extinction: the effect of environmental stress and lineage. Conservation Genetics 3,
301–307.
[Crossref]
Robertson
J.
&
Piercey-Normore
M.D. 2007.
Gene flow in symbionts of Cladonia arbuscula. Lichenologist 39,
69–82.
[Crossref]
Romeike
J.,
Friedl
T.,
Helms
G.
&
Ott
S. 2002.
Genetic diversity of algal and fungal partners in four species of Umbilicaria (lichenized ascomycetes) along a transect of the Antarctic Peninsula. Molecular Biology and Evolution 19,
1209–1207.
[Crossref]
Russell
G. J. 2006. Species richness v2.1. Available on the internet at http://www.eco-tools.net
Sancho
L.G.,
Schulz
F.,
Schroeter
B.
&
Kappen
L. 1999.
Bryophyte and lichen flora of South Bay (Livingston Island: South Shetland Islands, Antarctica). Nova Hedwigia 68,
301–337.
Schlensog
M.,
Pannewitz
S.,
Green
T.G.A.
&
Schroeter
B. 2004.
Metabolic recovery of continental Antarctic cryptogams after winter. Polar Biology 27,
399–408.
[Crossref]
Schmitt
I.,
Crespo
A.,
Divakar
P.K.,
Fankhauser
J.D.,
Herman-Sackett
E.,
Kalb
K.,
Nelsen
M.P.,
Nelson
N.A.,
Rivas-Plata
E.,
Shimp
A.D.,
Widhelm
T.
&
Lumbsch
H.T. 2009.
New primers for promising single-copy genes in fungal phylogenetics and systematics. Persoonia 23,
35–40.
[Crossref]
Skotnicki
M.L.,
Ninham
J.A.
&
Selkirk
P.M. 2000.
Genetic diversity, mutagenesis and dispersal of Antarctic mosses—a review of progress with molecular studies. Antarctic Science 12,
363–373.
[Crossref]
Smith
R.I.L. 1991.
Exotic sporomorpha as indicators of potential immigrant colonists in Antarctica. Grana 30,
313–324.
[Crossref]
Smith
R.I.L.
1993.
The role of bryophyte propagule banks in primary succession: case study of an Antarctic fellfield soil.
In J. Miles & D.W.H Walton (eds.): Primary succession on land. Pp. 55–78. Oxford: Blackwell.
Smith
R.I.L. 1994.
Vascular plants as bioindicators of regional warming in Antarctica. Oecologia 99,
322–328.
[Crossref]
Smith
R.C.
&
Stammerjohn
S.E.
1996.
Surface air temperature variations in the western Antarctic Peninsula region.
In R.M. Ross et al. (eds.): Foundations for ecological research west of the Antarctic Peninsula. Pp. 105–121. Washington, DC: American Geophysical Union.
Tajima
F. 1989.
Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 123,
585–595.
Templeton
A.,
Crandall
K.
&
Sing
C. 1992.
A cladistic analysis of phenotypic associations with haplotypes inferred from restriction endonuclease mapping and DNA sequence data. III. Cladogram estimation. Genetics 132,
619–633.
Tibell
L. 2001.
Photobiont association and molecular phylogeny of the lichen genus Chaenotheca. Bryologist 104,
191–198.
Turner
J.,
Colwell
S.R.,
Marshall
G.J,
Lachlan-Cope
T.A.,
Carleton
A.M.,
Jones
P.D.,
Lagun
V.,
Reid
P.A.
&
Lagovkina
S. 2005.
Antarctic climate change during the last 50 years. International Journal of Climatology 25,
279–294.
[Crossref]
Walser
J.C.,
Holderegger
R.,
Gugerli
F.,
Hoebee
S.E.
&
Scheidegger
C. 2005.
Microsatellites reveal regional population differentiation in Lobaria pulmonaria, an epiphytic lichen. Molecular Ecology 14,
457–467.
[Crossref]
Walser
J.C.,
Sperisen
C.,
Soliva
M.
&
Scheidegger
C. 2003.
Fungus-specific microsatellite primers of lichens: application for the assessment of genetic variation on different spatial scales in Lobaria pulmonaria. Fungal Genetics and Biology 40,
72–82.
[Crossref]
Werth
S.
&
Sork
V.L. 2010.
Identity and genetic structure of the photobiont of the epiphytic lichen Ramalina menziesii on three oak species in southern California. American Journal of Botany 97,
821–830.
[Crossref]
Wirtz
N.,
Lumbsch
H.T.,
Green
T.G.A.,
Türk
R.,
Pintado
A.,
Sancho
L.
&
Schroeter
B. 2003.
Lichen fungi have low cyanobiont selectivity in maritime Antarctica. New Phytologist 160,
177–183.
[Crossref]
Wirtz
N.,
Printzen
C.
&
Lumbsch
H.T. 2008.
The delimitation of Antarctic and bipolar species of neuropogonoid Usnea (Ascomycota, Lecanorales): a cohesion approach of species recognition for the Usnea perpusilla complex. Mycological Research 112,
472–484.
[Crossref]
Wynn-Williams
D.D. 1991.
Aerobiology and colonisation in Antarctica—the BIOTAS programme. Grana 30,
380–393.
[Crossref]
Zar
J.U.
1999. Biostatistical analysis. 4th edn. Englewood Cliffs, NJ: Prentice Hall.
Zoller
S.,
Lutzoni
F.
&
Scheidegger
C. 1999.
Genetic variation within and among populations of the threatened lichen Lobaria pulmonaria in Switzerland and implications for its conservation. Molecular Ecology 8,
2049–2059.
[Crossref]