RESEARCH/REVIEW ARTICLE
Tetraploids do not form cushions: association of ploidy level, growth form and ecology in the High Arctic Saxifraga oppositifolia L. s. lat. (Saxifragaceae) in Svalbard
Pernille Bronken Eidesen,1 Eike Müller,1 Christian Lettner,2 Inger Greve Alsos,3 Morgan Bender,4 Martin Kristiansen,5 Bart Peeters,6 Froukje Postma7 & Koen Frans Verweij8
1 Arctic Biology Department, University Centre in Svalbard, P.O. Box 156, NO-9171 Longyearbyen, Svalbard, Norway
2 Department of Conservation Biology, Vegetation Ecology and Landscape Ecology, Faculty Centre of Biodiversity, University of Vienna, Rennweg 14, AT-1030 Vienna, Austria
3 Department of Natural Sciences, Tromsø University Museum, NO-9037 Tromsø, Norway
4 School of Fisheries and Ocean Sciences, Institute of Arctic Biology, University of Alaska Fairbanks, P.O. Box 757500, AK 99775 Fairbanks, USA
5 Faculty of Biosciences, Fisheries and Economics, University of Tromsø, NO-9001 Tromsø, Norway
6 Faculty of Natural Sciences and Technology, Norwegian University of Science and Technology, Høgskoleringen 5, NO-7491 Trondheim, Norway
7 Department of Plant Ecology and Evolution, Faculty of Biology, Uppsala University, Norbyvägen 18 D, SE-752 36 Uppsala, Sweden
8 Community and Conservation Ecology Group, Faculty of Mathematics and Natural Sciences, University of Groningen, Nijenborgh 7, NL-9747 AG Groningen, The Netherlands
Abstract
Saxifraga oppositifolia L. is a common circumpolar plant species that displays considerable morphological and genetic variation throughout its range. It is mainly diploid, but tetraploids are reported from several regions. The growth form varies from prostate to cushion-shaped, and the plant thrives in wet snow beds as well as on dry ridges. This variation has triggered the curiosity of many researchers, but as yet, no one has explained the observed morphological variation using ecological and/or genetic factors. However, the ploidy level has rarely been taken into account. This is the first study that demonstrates a significant correlation between ploidy level, ecology and growth form in S. oppositifolia. We successfully analysed 193 individuals of S. oppositifolia from 15 locations in Svalbard to investigate possible relationships among growth forms (prostrate, intermediate and cushion), ecological factors (vegetation and soil characteristics) and ploidy level. Results from flow cytometry reported 106 diploids, eight triploids and 79 tetraploids. Tetraploids almost exclusively showed prostrate growth, while the diploids displayed all three growth forms, evidence that growth form is at least partly genetically determined. Our analyses of environmental and vegetation data in relation to ploidy level indicated overlapping niches, but the tetraploids showed a narrower niche, and one shifted towards more benign habitats characterized by higher pH, higher soil temperatures and higher cover of vascular plants. The latter may suggest that tetraploids are slightly better competitors, but less hardy. Thus, autopolyploidy in S. oppositifolia has expanded the ecological amplitude of this species complex.
Keywords
Autopolyploidy; flow cytometry; morphotypes; habitat segregation; purple saxifrage.
Correspondence
Pernille Bronken Eidesen, Arctic Biology Department, University Centre in Svalbard, P.O. Box 156, NO-9171 Longyearbyen, Svalbard, Norway.
E-mail: pernillee@unis.no
To access the supplementary material for this article, please see
supplementary files under Article Tools online.
(Published: 6 June 2013)
Polar Research 2013. © 2013 P.B. Eidesen et al. This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial 3.0 Unported License (http://creativecommons.org/licenses/by-nc/3.0/), permitting all non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Citation: Polar Research 2013, 32, 20071, http://dx.doi.org/10.3402/polar.v32i0.20071
Allopolyploids are polyploids that arise through hybridization between two different species, while an autopolyploid is a polyploid that arises from a single species. The formation of autopolyploids seems to be tightly linked with environmental fluctuations (Parisod et al. 2010). This link is evident in the Arctic, where large climatic oscillations and recurrent glaciations occurred throughout the Pleistocene (Dyke 2004; Svendsen et al. 2004). The Arctic is characterized by having many species with higher ploidy levels (Brochmann et al. 2004) and several ploidy levels within the same species due to autopolyploidy (Elven et al. 2012). The existence of different ploidy levels within a species is probably even more common than currently known. Because autopolyploids are usually morphologically similar to their diploid progenitors, they are often overlooked (Soltis et al. 2007; Parisod et al. 2010). However, although autopolyploids look similar, they may have different ecological preferences and dispersal abilities. Several autopolyploids have, for instance, shown successful range expansion and radiation (Soltis et al. 2007), indicating that genome doubling may represent an evolutionary advantage (Parisod et al. 2010). Thus, autopolyploidy can lead to the development of new, recognized species over time.
The purple saxifrage (Saxifraga oppositifolia L.) is a long-lived, insect-pollinated and mainly outcrossing herb (Kevan 1972; Gugerli 1998), with circumpolar Arctic–alpine distribution (Hultén & Fries 1986). It occupies a wide ecological amplitude and is one of the most hardy vascular plant species in the Arctic (Aiken et al. 2007). S. oppositifolia shows high morphological and genetic variation throughout the Arctic (Abbott et al. 2000; Holderegger et al. 2002; Abbott & Comes 2004; Elven et al. 2012; Müller et al. 2012; Winkler et al. 2012), and is known to harbour different ploidy levels (Elven et al. 2012). Both morphology and amplified fragment length polymorphism indicate that it is an autopolyploid (Elven et al. 2012; Müller et al. 2012). Also, there are no similar Saxifraga species in this part of the Arctic (Elven et al. 2012), which would seem to exclude an allopolyploid origin. Two main ploidy levels have been reported, 2n=26 and 2n=52, functionally diploid and tetraploid, with a few deviating numbers (Elven et al. 2012). Diploids have been reported from the entire distribution range (except Beringia), whereas tetraploids have been reported from Beringia, northern Canada, Greenland and Svalbard (Elven et al. 2012; Müller et al. 2012). In addition, triploids (3n=39) have been reported from one location in Greenland (together with two diploid counts; Jørgensen et al. 1958) and from two locations in Svalbard (Müller et al. 2012).
This variation has triggered the curiosity of many researchers and several subspecies have been suggested, but none fully agreed upon (Elven et al. 2012). Growth form, which varies from dense cushions to trailing plants, has been suggested to distinguish subspecies, but investigations of North American material (Aiken et al. 2005) and Svalbard material (Brysting et al. 1996), found the variation to be continuous and not useful as a diagnostic character. Based on several large-scale genetic studies, two major genetic groups have been detected (Abbott et al. 2000; Holderegger et al. 2002; Abbott & Comes 2004; Winkler et al. 2012) that may represent two subspecies (Elven et al. 2012). However, no one has managed to fully explain or relate the observed genetic variation with ecology and/or morphology, and none of these large-scale genetic studies has considered the ploidy level of investigated material.
Svalbard is a High-Arctic archipelago situated north-west of the Barents Sea, where the harsh climate provides prime conditions for S. oppositifolia. Two main growth forms of S. oppositifolia have been observed in Svalbard, cushions and prostrate, but intermediate forms do exist (Andersson & Hesselman 1900; Crawford et al. 1993; Rønning 1996). These different growth forms often grow in close spatial proximity, but previous studies in Svalbard have suggested that prostrate forms are more frequent in damp, moist and colder sites, whereas the compact cushion form is more common on dry, warm and exposed ridges (Crawford et al. 1993; Crawford et al. 1995; Brysting et al. 1996). A reinvestigation of taxonomic characters and ecological variation gave neither support for earlier suggested subspecies in Svalbard (ssp. reptans and pulvinata; see Rønning 1996) nor indication of ploidy differences based on pollen size (Brysting et al. 1996). The suggested correlation of growth form with ecology was also found to be vague, because morphological variation was continuous along local ecological gradients (Brysting et al. 1996).
However, in a recent study by Müller et al. (2012), two main genetic groups of S. oppositifolia in Svalbard were identified based on amplified fragment length polymorphisms (AFLP) fingerprinting. Based on flow cytometry analyses of a subset of plants representing these two AFLP groups, it was shown that the genetic groups corresponded to diploid and tetraploid individuals. It was assumed that the origin of the tetraploid was a single event, as only one tetraploid genetic group was discovered. Different ploidy levels grew intermixed, but some purely di- or tetraploid sampling sites were found. The data showed no difference in dispersal ability (Müller et al. 2012); these monotypic sampling sites may indicate some habitat difference between ploidy levels. However, Müller et al. (2012) did not collect ecological data that could confirm this hypothesis, and no analyses were made of morphology that could associate ploidy level with growth form.
In this study, we aimed to investigate the possible association of ploidy, ecology and growth form in S. oppositifolia in Svalbard by: (i) testing whether ploidy levels are related to the observed growth forms and (ii) evaluating whether diploids and tetraploids have different ecological amplitudes.
Material and methods
Study area and sampling
Svalbard is a High-Arctic archipelago situated between 74°–81°N and 10°–35°E. S. oppositifolia is likely the most widely distributed vascular plant in Svalbard, and is often dominant in the vegetation (Elvebakk 1994; Alsos et al. 2012). Samples of S. oppositifolia were collected from 15 locations, covering all three bioclimatic zones present in Svalbard—the middle-Arctic tundra zone, the northern Arctic tundra zone and the Arctic polar desert zone (Elvebakk 2005; Walker et al. 2005)—and from different vegetation types, to cover the intraspecific variation in ecology and growth form. The collection period was from mid-July to mid-August 2011. Usually, we had one sampling site within each location. In five locations, we had two sampling sites: one in a ridge habitat and one in a slope habitat (Table 1; Fig. 1). In total, leaf material from 195 individuals was collected for ploidy analysis. Most material was dried in silica gel, but some samples were analysed fresh. The growth forms were classified subjectively by the observer into cushion type (the individual plant formed a dense cushion), intermediate type (the individual plant formed dense, cushion-like parts, but also trailing branches) and prostrate type (the individual plant had only trailing parts). In the immediate vicinity of 169 of the 195 sampled individuals, environmental and vegetation data were collected (Table 1). The distance between sampled individuals was usually 10 m but in some cases less, the minimum distance was 5 m. Vouchers of all sampled individuals are stored at the University Centre in Svalbard.
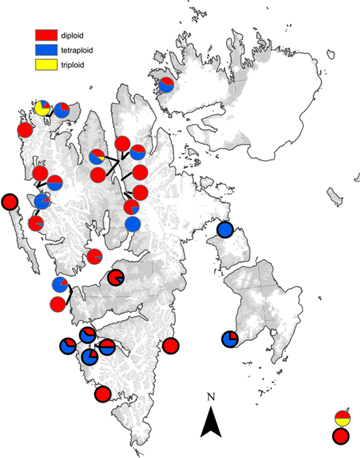
Fig. 1
Distribution of diploid, triploid and tetraploid individuals of Saxifraga oppositifolia in Svalbard based on 193 samples analysed by flow cytometry viewed as propositions within the 20 sampling sites analysed in this study (Table 1), and selected samples from Müller et al. 2012 (encircled in black), where ploidy levels were inferred from amplified fragment length polymorphisms patterns in combination with flow cytometry.
Table 1 Overview of the 20 sampling sites at 15 locations in Svalbard where 193 of 195 collected samples of Saxifraga oppositifolia were successfully analysed for flow cytometry analyses.
| Location |
Region |
Area |
Locality |
Vegetation typea
|
Latitude N |
Longitude E |
No.b
|
Plotsc
|
pH |
2xd
|
3xd
|
4xd
|
cd
|
id
|
pd
|
| 1 |
Haakon VII Land |
Krossfjorden |
14. Julibukta |
Bird cliff, moss tundra, slope |
79°07.694 |
11°51.360 |
7 |
6 |
7.76 |
6 |
– |
1 |
4 |
3 |
– |
|
|
Krossfjorden |
14. Julibukta |
Bird cliff, moss tundra, ridge |
79°07.724 |
11°51.277 |
8 |
8 |
6.98 |
8 |
– |
– |
2 |
5 |
1 |
| 2 |
Nordenskiöld Land |
Isfjorden |
Kapp Linné, Isfjord Radio |
Mesic Luzula nivalis tundra, slope |
78°03.561 |
13°38.261 |
7 |
7 |
6.36 |
1 |
– |
6 |
– |
4 |
3 |
|
|
Isfjorden |
Kapp Linné, Isfjord Radio |
Mesic Luzula nivalis tundra, ridge |
78°03.544 |
13°38.273 |
8 |
7 |
7.28 |
8 |
– |
– |
8 |
– |
– |
| 3 |
Ny-Friesland |
Raudfjorden |
Alicehamna |
Mesic Luzula confusa tundra,ridge |
79°44.509 |
12°17.333 |
7 |
5 |
7.81 |
2 |
– |
5 |
2 |
– |
5 |
|
|
Raudfjorden |
Alicehamna |
Mesic Luzula confusa tundra, slope |
79°44.397 |
12°14.343 |
7 |
7 |
6.69 |
1 |
5 |
1 |
2 |
– |
5 |
| 4 |
|
Wijdefjorden |
Ringhorndalen |
Cassiope tetragona tundra, slope |
79°19.978 |
16°06.960 |
7 |
6 |
7.33 |
3 |
– |
4 |
– |
– |
7 |
|
|
Wijdefjorden |
Ringhorndalen |
Cassiope tetragona tundra, ridge |
79°20.023 |
16°06.960 |
7 |
5 |
8.46 |
6 |
– |
– |
5 |
1 |
– |
| 5 |
|
Wijdefjorden |
Coast plain, outside Ringhorndalen |
Potentilla pulchella steppes, slope |
79°18.699 |
15°57.796 |
10 |
10 |
8.46 |
4 |
1 |
5 |
3 |
– |
7 |
|
|
Wijdefjorden |
Coast plain, outside Ringhorndalen |
Potentilla pulchella steppes, ridge |
79°18.744 |
15°55.524 |
5 |
5 |
8.05 |
5 |
– |
– |
5 |
– |
– |
| 6 |
|
Austfjorden |
Austbotnhytta |
Potentilla pulchella steppes |
78°59.324 |
16°22.329 |
5 |
– |
– |
4 |
– |
– |
4 |
– |
– |
| 7 |
|
Austfjorden |
Bjørnnesholmen |
Potentilla pulchella steppes |
79°07.886 |
16°06.196 |
5 |
– |
– |
5 |
– |
– |
5 |
– |
– |
| 8 |
|
Austfjorden |
Gyllensköldholmane |
Potentilla pulchella steppes |
79°00.205 |
16°15.951 |
5 |
– |
– |
4 |
– |
1 |
3 |
1 |
1 |
| 9 |
Oscar II Land |
Isfjorden |
Bohemanflya |
Cassiope tetragona tundra |
78°23.417 |
14°44.433 |
18 |
18 |
6.5 |
17 |
– |
1 |
12 |
2 |
4 |
| 10 |
|
Forlandsundet |
Engelskbukta |
Cassiope tetragona tundra |
78°51.066 |
11°48.033 |
16 |
16 |
6.3 |
15 |
– |
1 |
4 |
3 |
9 |
| 11 |
|
Kongsfjorden |
Midtre Lovénbreen |
Mesic Luzula nivalis tundra |
78°54.510 |
12°05.103 |
20 |
20 |
7.7 |
2 |
– |
18 |
– |
– |
20 |
| 12 |
Albert I Land |
Magdalenefjorden |
Gravneset |
Mesic Luzula confusa, ridge |
79°35.434 |
10°59.779 |
9 |
9 |
4.9 |
9 |
– |
– |
2 |
3 |
4 |
| 13 |
Dickson Land |
Billefjorden |
Petuniabukta, Skottehytta |
Dry Dryas octopetala tundra |
78°41.946 |
16°37.018 |
30 |
30 |
7.7 |
– |
– |
30 |
– |
– |
30 |
| 14 |
Hopen |
Hopen |
Hopen Meteorological Station |
Papaver dahlianum polar desert |
76°30.443 |
25°00.622 |
4 |
– |
– |
2 |
2 |
– |
– |
4 |
– |
| 15 |
Gustav V Land |
Murchisonfjorden |
Florabukta |
Manured polar desert |
80°01.816 |
18°41.650 |
10 |
10 |
6.4 |
4 |
– |
6 |
5 |
– |
5 |
| aMain vegetation type, following Elvebakk (2005). |
| bNumber of samples collected for ploidy analyses. |
| cNumber of vegetation plots analysed per sampling site. |
| dPloidy level and growth form are indicated as number of diploids (2×), triploids (3×) and tetraploids (4 x) and cushion (c), intermediate (i) and prostrate (p). |
Collection of vegetation and environmental data
Vascular plant species were recorded using the point frame intercept method (Bråthen & Agberg 2004) with a 0.25 m2 frame and 25 equidistant points. In addition, cover percentages of surface types were estimated (bryophytes, lichens, soil biological crust, litter and bare ground). Vascular plant species that were not recorded with the intercept method, but that occurred within the frame, were noted.
Soil moisture and soil temperature were measured at 5 cm depth in each plot. The soil moisture and soil temperature at location 9, 10, 11,13 and 15 (Table 1) were measured with a HOBO Micro Station Data Logger H21–002 with 2/2 Temp/RH sensors (MicroDAQ.com, Ltd., Contoocook, NH, USA). For the other plots, a digital thermometer was used to measure soil temperature, and moisture was estimated by appearance and feel of the soil (e.g., Klocke & Fischbach 1984). The soil moisture measurements were later transformed to classes to enable comparability to the estimated soil moisture. Soil samples were taken at 0–5 cm depth to determine pH, conductivity and loss on ignition (LOI). These samples were stored at 4°C until further processing.
Soil pH, conductivity and LOI determination
The soil samples from each sampling site were pooled and sieved with a 2-mm meshed sieve. From each of the pooled and sieved soil samples, we took sub-samples for pH, conductivity measurements and LOI determination. The soil used for pH and conductivity measurements was oven-dried at 31.8°C for 24 h. Thereafter, 10 g soil was mixed with 25 ml distilled water, shaken and left to settle for 12 h. Measurements were taken with a VWR sympHony SP90M5 with a pH electrode and a conductivity electrode (VWR International, Radnor, PA, USA).
For the LOI determination, we followed the procedures described by Dean (1974) and improved by Heiri et al. (2001). Three sub-samples from each sampling site were oven-dried for 24 h at 105°C, then oven-burned for 4 h at 550°C. We ensured that the weight of each soil sample replicate was similar, as Heiri et al. (2001) showed that LOI at 550°C is dependent on sample size. The percentage LOI550 was calculated as LOI550=((DW105–DW550)/DW105)*100 (Heiri et al. 2001).
Ploidy analysis
All ploidy analyses were completed by Plant Cytometry Services, AG Schijndel, The Netherlands, using the following protocol: each leaf sample was prepared by chopping 20–50 mg with a razor blade in an ice-cold buffer containing 5 mM HEPES, 10 mM magnesium sulphate heptahydrate, 50 mM potassium chloride, 0.2% Triton X-100, 0.1% dithiothreitol, 1% polyvinylpyrrolidone and 2 µg/ml DAPI; buffer pH was 8.0. Approximately 2 ml of the solution containing cells and tissue were passed through a nylon filter of 50-µm mesh size. Fluorescence was measured with a CyFlow ML flow cytometer (Partec GmbH, Münster, Germany). The flow cytometer was equipped with a high-pressure mercury lamp (Osram HBO 100 long life), heat protection filter KG-1, excitation filters UG-1 and BG-38, dichroic mirrors TK 420 and TK 560 and an emission filter GG 435. In addition, FlowMax software (version 2.4, Partec GmbH, Münster, Germany) was used.
Data analysis
The measured ploidy levels (diploid, triploid and tetraploid) were sorted according to the registered growth forms (cushion, intermediate and prostrate) and analysed for association in a contingency table applying the program PAST version 2.15 (Hammer et al. 2001). A contingency table was also used to test the association between habitats (ridge or slope) and ploidy level and growth form.
In addition, the results from the triploid counts were removed and the remaining frequencies were tested applying a χ2 test. Further, the ploidy and growth form data were fitted to a generalized linear model using R for Mac version 2.14.0 (R Development Core Team 2011), where the observed counts of the different ploidy and growth form combinations were the response variables and ploidy and growth form were the predictor variables. Best model fit was achieved by specifying the model with a negative binomial error distribution. In order to characterize the habitats and ecological amplitude of S. oppositifolia, point frame data of the vegetation around or in the vicinity of each sample, and the associated environmental variables, were analysed with ordination techniques applying the R package Vegan 2.0–3 (Oksanen et al. 2012). Three basic ordination methods were applied: non-metric multidimensional scaling (NMDS), principal component analysis and detrended correspondence analysis (DCA). Spearman's rank correlation coefficients were determined for all environmental variables that had a significant influence in the preferred ordination analysis. In addition, these variables were tested for significant differences between the two main ploidy levels and the three growth forms. For the differences according to ploidy levels, a Wilcoxon signed-rank test was used, and for differences according to growth forms, a Kruskal–Wallis one-way analysis of variance was applied. The series-packing module in PAST (Hammer et al. 2001) was used to fit Gaussian response models to the abundances of different ploidy levels of S. oppositifolia along each of the environmental gradients showing significant differences between ploidy level and/or growth form. In these analyses, it was assumed that all point frame hits of S. oppositifolia within a vegetation plot had the same ploidy level and growth form as the sampled plant with which the vegetation plot was associated.
Results
Distribution of ploidy levels and growth forms
Ploidy level could be successfully determined in 193 of the 195 collected samples. Our results reconfirmed the presence of three ploidy levels in S. oppositifolia in Svalbard, with a majority of diploids and tetraploids (see Müller et al. 2012; Fig. 1): 106 (55%) were diploids, eight (4%) were triploids and 79 (41%) were tetraploids. The geographical distribution of ploidy levels showed that both diploids and tetraploids are widespread in Svalbard, and no clear overall distribution pattern was found (Fig. 1). Diploids and tetraploids co-occurred within 11 sampling sites, while eight sampling sites were monotypic (Table 1). The different growth forms also seemed to be widespread and often intermixed, but separate analyses of the locations where both ridge and slope habitats were sampled showed that ploidy level (χ2=16.83, df=2; P<0.0002) and growth form (χ2=14.494, df=2; P<0.001) were strongly related to habitat. Tetraploids and the prostrate growth form were clearly more frequent in slope habitats, while the diploids and the cushion growth form were clearly more frequent on ridges (Table 2).
Table 2 Distribution of observed ploidy level and growth form of 72 Saxifraga oppositifolia individuals in Svalbard distributed between slope and ridge habitats. Both habitats were collected in five different locations.
| |
Ridge |
Slope |
| Diploid |
29 |
15 |
| Triploid |
0 |
6 |
| Tetraploid |
5 |
17 |
| Cushion |
22 |
9 |
| Intermediate |
6 |
7 |
| Prostrate |
6 |
22 |
Relations between ploidy level and growth form
While all three growth forms were observed among diploids, no cushion forms were observed in triploids, and only two of the 79 tetraploids, were reported as cushion-shaped (Table 3). The frequency distributions of growth forms and ploidy levels were significantly different in all three performed tests. For the full contingency table with all growth forms and ploidy levels, Cramér's V was 0.508 with χ2=99.596 (df=4; P<0.0001). The deviations from independence of rows and columns in the two dimensional contingency table are shown in the Cohen-Friendly association plot (Fig. 2). After removing the triploid data, the χ2=94.357 (df=2; P<0.0001). The results from the generalized linear model confirmed the two former tests, and showed a significant difference in the count of diploids and tetraploids forming cushions. Further, the model showed a significantly higher count in prostrate tetraploids (Table 4).
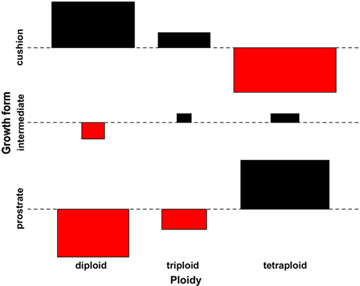
Fig. 2
Cohen-Friendly association plot of ploidy levels and growth forms of 193 Saxifraga oppositifolia samples in Svalbard indicating deviations from independence. Black bars show the excess of diploid cushions and tetraploid prostrate plants, red bars show categories where fewer individuals were observed than expected under the null hypothesis of independence of growth form and ploidy.
Table 3 Distribution of observed ploidy level and growth form of 193 Saxifraga oppositifolia individuals in Svalbard.
| Ploidy/growth form |
Diploid |
Triploid |
Tetraploid |
| Cushion |
64 |
0 |
2 |
| Intermediate |
20 |
2 |
4 |
| Prostrate |
22 |
6 |
73 |
Table 4 Output of the generalized linear model with negative binomial error distribution based on 193 Saxifraga oppositifolia individuals of different ploidy level and growth form in Svalbard.
| |
|
Estimate |
Standard error |
z |
p |
| Ploidy |
(Intercept) |
3.060 |
0.636 |
4.809 |
0.000 |
|
Tetraploid |
−3.466 |
1.138 |
−3.046 |
0.002 |
|
Triploid |
−22.363 |
5442.460 |
−0.004 |
0.997 |
| Growth form |
Intermediate |
−1.163 |
0.919 |
−1.266 |
0.206 |
|
Prostrate |
−1.068 |
0.916 |
−1.165 |
0.244 |
| Interaction |
Tetraploid: intermediate |
1.856 |
1.541 |
1.205 |
0.228 |
|
Triploid: intermediate |
20.060 |
5442.460 |
0.004 |
0.997 |
|
Tetraploid: prostrate |
4.665 |
1.460 |
3.195 |
0.001 |
|
Triploid: prostrate |
21.064 |
5442.460 |
0.004 |
0.997 |
Analyses of environmental variables related to ploidy level and growth form
All ordination methods showed similar results, but we gave preference to the NMDS. This method has the advantage over other ordination techniques in that it makes only a few assumptions about the nature of the data, for example, species responses are not restricted to linear relationships (Oksanen 2011). Some of the measured environmental variables had no significant influence (soil moisture) or very low influence (R2 below 0.1; bare ground and litter) on the vegetation structure. Based on the NMDS, the most relevant environmental variables structuring the vegetation were soil temperature, lichen cover, LOI, pH and bryophyte cover (Supplementary Table S1). All environmental variables were further tested for differences between plots with different ploidy levels and different growth forms. Lichen cover, bare ground, soil pH, soil temperature and vascular plant cover were significantly different between plots with different ploidy level and different growth form. Cover of litter was only significantly different between plots with different growth form (Supplementary Table S2). The correlation between environmental variables was high for several variables (Supplementary Table S3). Soil pH was closely negatively correlated to lichen cover and LOI (Rs=−0.59, P<0.001; Rs=−0.50, P<0.001). Bare ground and vascular plant cover were also strongly negatively correlated (Rs=−0.55, P<0.001).
A NMDS plot was drawn including all environmental variables (Fig. 3). Two sampling sites in Wijdefjorden (Location 5; Table 1) had a strong deviation in species composition compared to the other sampling sites and were excluded from the final multivariate analysis in order to achieve a better resolution of the remaining sampling sites. The NMDS plot clearly showed an ecological difference between ploidy levels. Vegetation plots associated with tetraploids were clustered together, while the diploids appeared more scattered. The micro-habitat associated with tetraploids was clearly characterized by higher soil pH, higher vascular plant cover and higher soil temperatures. The few scattered tetraploids situated at the lower end of NMDS axis 1, were samples from Florabukta. Some of these samples were collected close to a bird cliff, and were the only vegetation plots sampled in manured polar desert.
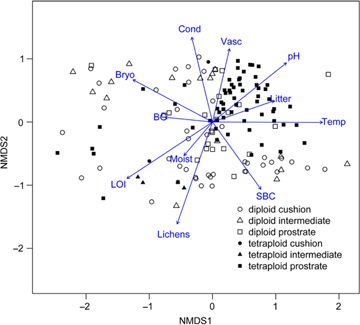
Fig. 3
Non-metric multidimensional scaling (NMDS) plot showing 148 vegetation plots where diploid and tetraploid individuals of Saxifraga oppositifolia were sampled in Svalbard, including 11 fitted environmental variables. Plots with triploids, plots without vascular vegetation cover and plots from two sampling sites in Wijdefjorden (Location 5; Table 1), which had a strongly deviating species composition compared to the other sampling sites, were excluded from the presented multivariate analysis, in order to achieve a better resolution of the remaining sites. The following terms have been abbreviated: bare ground (BG), bryophyte cover (Bryo), conductivity (Cond), lichen cover (Lichens), coverage of litter (Litter), loss on ignition (LOI), soil moisture (Moist), soil pH (pH), soil biological crust (SBC), soil temperature (Temp) and vascular plant cover (Vasc).
Gaussian response models of diploids versus tetraploids reflected the ecological differences found in other analyses. The environmental variables showing differences in optimum values for diploids versus tetraploids were soil pH (6.53 vs. 7.65; Fig. 4), soil temperature (8.8°C vs. 12.0°C), bare ground (21.2% vs. 4.0%) and vascular plant cover (29.4% vs. 46.3%). Therefore, diploids seemed to prefer lower pH, less vegetation cover and lower temperatures compared to the tetraploids. Diploids showed also higher tolerance for all tested environmental variables (soil pH=±1.14 vs. ±0.24; Fig. 4; soil temperature=±3.1°C vs. ±2.2°C; bare ground; ±28.1% vs. ±9.2%; vascular plant cover=±15.9% vs. ±14.4%). Doing the same analyses sorting by growth form (cushions versus prostrate) gave mainly the same values and results. Cushions preferred more bare ground, less vegetation cover and lower temperatures and had overall higher tolerance levels. The exception was for soil pH, where only minor differences were found between growth forms (7.34±0.73 vs. 7.48±0.62).
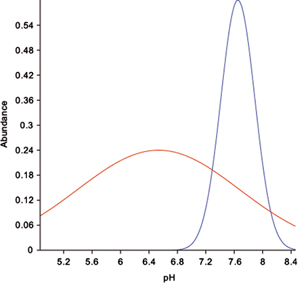
Fig. 4
A Gaussian response model fitted to the abundance of diploids (red line) and tetraploids (blue line) of Saxifraga oppositifolia in 163 vegetation plots along a gradient of soil pH (six plots with triploids discarded). The fitted parameters are optimum pH (average: 6.53 vs. 7.65), pH tolerance (standard deviation:±1.14 vs. 0.24) and maximum abundance of the two ploidy levels (0.24 vs. 0.6).
Discussion
The two main growth forms of S. oppositifolia (cushion and prostrate) in Svalbard were recognized early (Andersson & Hesselman 1900), as well as their common occurrence in close spatial proximity (Crawford et al. 1993; Crawford et al. 1995; Brysting et al. 1996). While it has been assumed by Rønning (pers. comm. in Crawford et al. 1993) that the prostrate plants in Svalbard were tetraploid and the more compact ones were diploid, Brysting et al. (1996) were not able to confirm such an association with their data. Here, we clearly show that there is a strong link between growth form, ploidy level and ecological amplitude in S. oppositifolia in Svalbard (Figs. 2–4).
Diploids are able to express all three growth forms, but they are predominantly cushion-shaped, while tetraploids exhibit almost exclusively prostrate growth (Fig. 2; Table 3). Our results provide some support for the assumption that the growth form of tetraploids is determined genetically (Brysting et al. 1996), and may explain the observed stability of growth form in transplant experiments (Crawford et al. 1995). However, for the diploids, which express all three growth forms, an interaction between genetic and environmental factors may determine growth form.
Although diploids and tetraploids co-occurred in most locations, very few triploids (4%) were recorded. It was therefore not possible to evaluate associations between triploids and growth form or ecology (Table 1). The low number of triploids found in our study may suggest the presence of reproductive barriers between diploids and tetraploids. Based on AFLP markers, Müller et al. (2012) showed that S. oppositifolia in Svalbard were structured into two genetic groups representing diploids and tetraploids, while no geographical structure was detected. This lack of geographical structure clearly indicated limited gene flow between ploidy levels (Müller et al. 2012). A study of a mixed population of Chamerion angustifolium, with tetraploids (55%), diploids (36%) and triploids (9%), indicated that flowering phenology, with diploids flowering earlier, had a significant effect on prezygotic mating isolation and triploid production (Husband & Schemske 2000). Our data show that diploids are more frequent in habitats with less snow cover (ridges), which usually melts out early in spring. Thus, different phenology and flowering time between ploidy levels might contribute to prezygotic mating isolation in S. oppositifolia as well. However, as also suggested by Müller et al. (2012), the presence of triploids does suggest that some gene flow among ploidy levels may occur, but whether it is sufficient to keep the ploidy levels as one species over time, is a question still to be answered.
The former lack of evidence to verify an association between growth form and ploidy level can be explained by the combination of small sample sizes, lack of efficient ploidy estimation techniques and, most importantly, the fact that the relationship of ploidy and growth form is not mutually exclusive. One possible (simplified) genetic explanation of the observed relation between ploidy level and growth form is that if prostrate growth is dominantly expressed over cushion growth, and the ancestor of the tetraploid lineage was dominant homozygote for prostrate growth, the newly originated tetraploid would lose the ability to grow cushions. As there is some gene flow between the ploidy levels (Müller et al. 2012), the tetraploids may restore some ability to form cushions over time.
Another possibility is that growth form is linked to genes related to various ecological adaptations, which differ between ploidy levels. Both ploidy levels were widespread and occurred intermixed in several habitat types, suggesting wide, and largely overlapping, ecological amplitudes of both diploids and tetraploids. Such coexistence of sympatric ploidy levels requires highly specialised small-scale habitat and niche differentiation between them (Thompson & Lumaret 1992). However, these small-scale habitat differences can be difficult to decipher, especially as such small-scale ecological variables can be overruled by large-scale differences like bedrock and oceanic influences (Elvebakk 2005; Walker et al. 2005). For example, the presence of a stronger ecological variable on a larger scale might explain the eight monotypic sampling sites revealed in this study.
When looking into the distribution of ploidy levels and growth forms between slope and ridge habitats, it is evident that tetraploids (and prostrate growth forms) are more commonly associated with slope habitats, while the diploids (and cushion growth forms) prefer ridges. This supports earlier studies suggesting that prostrate forms are more frequent in damp, moist and colder sites, whereas the compact cushion form prefers dry, warm and exposed ridges (Crawford et al. 1993; Crawford et al. 1995; Brysting et al. 1996). Further analyses of environmental and vegetation data based on NMDS and Gaussian response models showed that the tetraploids preferred higher soil pH, higher soil temperatures and higher vegetation cover compared to diploids (Figs. 3, 4). The preferences regarding vegetation cover are in accordance with the earlier assumptions mentioned above and fit with the typical ridge and slope habitats in Svalbard (Elvebakk 1999). The results related to soil temperature are contradictory to earlier suggestions (Crawford et al. 1993; Crawford et al. 1995; Brysting et al. 1996). However, our temperature results must be interpreted with care. Although soil temperature showed clear differences between ploidy levels and growth forms, temperatures were registered as a point measurement and may have been strongly influenced by the weather conditions on the given sampling day. Nevertheless, our temperature results fit well to the mortality data in Crawford et al. (1995), where the prostrate form had a significant higher mortality at a given lower temperature (10°C) than the compact cushion form.
A preference for slope habitats versus ridge habitats indirectly suggests that the tetraploids prefer snow cover during winter. The annual distribution and duration of snow is strongly dependent on topography and is of great importance for small-scale vegetation structure in the Arctic (Billings & Mooney 1968; Elberling et al. 2008). Ecological preferences related to different ploidy levels can therefore vary over very small distances, e.g., in areas with patterned ground. This may explain the apparently intermixed appearance of growth forms and/or ploidy levels.
S. oppositifolia has been reported to be a circum-neutral species, with pH ranging from 7.43 to 6.13 (Elvebakk 1982). The registered tolerance for diploids in our study was much wider, and the optimum registered for tetraploids was even outside this range (7.65). Further, tetraploids seem to have a narrower pH range than diploids. As edaphic variables like pH might vary on a larger scale (e.g., Walker et al. 2005), pH or edaphic variables linked to pH may explain the occurrence of monotypic sampling sites.
Surprisingly, no difference in optimum tolerance for pH was found between growth forms, even though pH showed significant association with growth form when comparing plots (Supplementary Table S2). As diploids can express all growth forms, a reason for this pattern could be that pH preference and tolerance are directly linked to ploidy level rather than growth form. In addition, the strong connection between prostrate growth and tetraploids could lead to a false significant association between growth form and pH.
Although diploid S. oppositifolia seems to prefer ridge type of habitats, it can cope with a large range of habitats (Figs. 3, 4). The diploids had higher tolerance than tetraploids for all tested environmental variables. The establishment of the tetraploid lineage within S. oppositifolia has created a lineage with an overall narrower—but shifted—ecological optimum towards more alkaline soil and higher vegetation cover (Figs. 3, 4). The latter may indicate that the tetraploid is a slightly better competitor than the diploid. Even though the tetraploid has a narrower range, the origin of this tetraploid lineage has most likely increased the range of the species sensu lato in Svalbard, and probably also in other regions as this tetraploid lineage most likely originated outside Svalbard (Müller et al. 2012). These results confirm the frequently observed pattern that polyploidization within species complexes expands their ecological amplitudes (Bayer 1999; Soltis et al. 2004; Treier et al. 2009), which is in line with the predominance of polyploids in the Arctic (Brochmann et al. 2004).
Our results further highlight the importance of including ploidy level analyses in the interpretation of a species evolutionary history. S. oppositifolia has been regarded as a model-species in Arctic–alpine plant evolution and phylogeographical research (Holderegger & Abbott 2003; Winkler et al. 2012). Many researchers have tried to untangle the history of this species through different molecular marker systems (Abbott et al. 2000; Holderegger et al. 2002; Holderegger & Abbott 2003; Abbott & Comes 2004; Winkler et al. 2012). Common factors to all these large-scale studies is that no more than two major, supported groups of genotypes have been detected, and that none of them have evaluated the ploidy levels of their samples. It is worthwhile to notice that the most recent investigation supporting two lineages was based on AFLPs and chloroplast sequences (Winkler et al. 2012), and that AFLP patterns have been shown to clearly reflect ploidy levels in S. oppositifolia (Müller et al. 2012). The perception that S. oppositifolia is predominantly diploid outside Beringia (Elven et al. 2012) may not be correct.
Conclusion
This is the first study that demonstrates a significant correlation between ploidy level, growth form and ecology in S. oppositifolia. Growth form is at least partly determined genetically, as tetraploids are strongly linked with prostrate growth. Diploids have a preference for ridge habitats, but show an overall much wider ecological niche and phenotypic diversity than tetraploids. Although tetraploids display a narrower niche, it is slightly shifted towards more alkaline habitats with higher vegetation cover. Thus, autopolyploidy within S. oppositifolia has led to an expansion of the species’ ecological niche.
Acknowledgements
Sincere thanks to all participants in the AB-326 Arctic Plant Ecology course and AB-201 Terrestrial Arctic Biology course at UNIS 2011 for their help with fieldwork and laboratory work, and to Ahti Launis for field collections. CL gratefully acknowledges financial support from the Österreichische Gesellschaft für Polarforschung and a grant from the Siegfried Ludwig-Fonds compensating travel expenses.
References
Abbott
R.J.
&
Comes
H.P. 2004.
Evolution in the Arctic: a phylogeographic analysis of the circumarctic plant, Saxifraga oppositifolia (purple saxifrage). New Phytologist 161,
211–224.
Publisher Full Text
Abbott
R.J.,
Smith
L.C.,
Milne
R.I.,
Crawford
R.M.M.,
Wolff
K.
&
Balfour
J. 2000.
Molecular analysis of plant migration and refugia in the Arctic. Science 289,
1343–1346.
PubMed Abstract | Publisher Full Text
Aiken
S.G.,
Dallwitz
M.J.,
Consaul
L.L.,
McJannet
C.L.,
Boles
R.L.,
Argus
G.W.,
Gillett
J.M.,
Scott
P.J.,
Elven
R.,
LeBlanc
M.C.,
Gillespie
L.J.,
Brysting
A.K.,
Solstad
H.
&
Harris
J.G. 2007. Flora of the Canadian Arctic Archipelago: descriptions, illustrations, identification, and information retrieval. Ottawa: NRC Research Press, National Research Council of Canada, Ottawa. Accessed on the internet at http://nature.ca/aaflora/data on 30 April 2013.
Aiken
S.G.,
LeBlanc
M.C.
&
Boles
R.L. 2005.
Growth forms and sepal hairs of the purple saxifrage (Saxifraga oppositifolia: Saxifragaceae) in North America related to chromosome records and DNA information. Canadian Journal of Botany 83,
1088–1095.
Publisher Full Text
Alsos
I.G.,
Elven
R.,
Sandbakk
B.E.
&
Arnesen
G. 2012. The flora of Svalbard. Accessed on the internet at http://svalbardflora.net on 9 March 2012.
Andersson
G.
&
Hesselman
H. 1900. Bidrag till kännedomen om Spetsbergens och Beeren Eilands kärlväxtflora, grundade på iakttagelser under 1898 års svenska polarexpedition. (Contribution to the knowledge of Spitsbergen's and Bjørnøya's vascular plants, based on observations during the 1898 Swedish polar expedition.) Bihang till Kungliga Svenska Vetenskaps-akademiens Handlingar 26. Stockholm: Royal Swedish Academy of Sciences.
Bayer
R. 1999.
New perspectives into the evolution of polyploid complexes.
In L.W.D. van Raamsdonk & J.C.M. den Nijs (eds.): Plant evolution in man-made habitats. Proceedings of the VIIth international symposium of the international organization of plant biosystematists. Pp. 359–373. Amsterdam: Hugo de Vries Laboratory.
Billings
W.D.
&
Mooney
H.A. 1968.
The ecology of Arctic and alpine plants. Biological Reviews 43,
481–529.
Publisher Full Text
Bråthen
K.A.
&
Agberg
O. 2004.
More efficient estimation of plant biomass. Journal of Vegetation Science 15,
653–660.
Publisher Full Text
Brochmann
C.,
Brysting
A.K.,
Alsos
I.G.,
Borgen
L.,
Grundt
H.H.,
Scheen
A.C.
&
Elven
R. 2004.
Polyploidy in Arctic plants. Biological Journal of the Linnean Society 82,
512–536.
Publisher Full Text
Brysting
A.K.,
Gabrielsen
T.M.,
Sørlibråten
O.,
Ytrehorn
O.
&
Brochmann
C. 1996.
The purple saxifrage, Saxifraga oppositifolia, in Svalbard: two taxa or one? Polar Research 15,
93–105.
Publisher Full Text
Crawford
R.M.M.,
Chapman
H.M.,
Abbott
R.J.
&
Balfour
J. 1993.
Potential impact of climatic warming on Arctic vegetation. Flora 188,
367–381.
Crawford
R.M.M.,
Chapman
H.M.
&
Smith
L.C. 1995.
Adaptation to variation in growing season length in Arctic populations of Saxifraga oppositifolia L. Botanical Journal of Scotland 47,
177–192.
Publisher Full Text
Dean
W.E. 1974.
Determination of carbonate and organic matter in calcareous sediments and sedimentary rocks by loss on ignition; comparison with other methods. Journal of Sedimentary Petrology 44,
242–248.
Dyke
A.S. 2004.
An outline of North American deglaciation with emphasis on central and northern Canada.
In J. Ehlers & P.L. Gibbard (eds.): Quaternary glaciations: extent and chronology. Pp. 373–424. Amsterdam: Elsevier.
Elberling
B.,
Tamstorf
M.P.,
Michelsen
A.,
Arndal
M.F.,
Sigsgaard
C.,
Illeris
L.,
Bay
C.,
Hansen
B.U.,
Christensen
T.R.,
Hansen
E.S.,
Jakobsen
B.H.
&
Beyens
L. 2008.
Soil and plant community-characteristics and dynamics at Zackenberg.
In H. Meltofte et al. (eds.): High-Arctic ecosystem dynamics in a changing climate. Pp. 223–248. London: Academic Press.
Elvebakk
A. 1982.
Geological preferences among Svalbard plants. Inter-Nord 16,
11–31.
Elvebakk
A. 1994.
A survey of plant associations and alliances from Svalbard. Journal of Vegetation Science 5,
791–802.
Publisher Full Text
Elvebakk
A. 1999. Bioclimatic delimitation and subdivision of the Arctic. In
I.
Nordal
&
V.Y.
Razzhivin (eds.): The species concept in the high north: a panarctic flora initiative. Pp. 81–112.
Oslo: Norwegian Academy of Science and Letters.
Elvebakk
A. 2005.
A vegetation map of Svalbard on the scale 1:3.5 mill. Phytocoenologia 35,
951–967.
Publisher Full Text
Elven
R.,
Murray
D.F.,
Razzhivin
V.Y.
&
Yurtsev
B.A. 2012. Annotated checklist of the panarctic flora (PAF) vascular plants. Accessed on the internet at http://nhm2.uio.no/paf/ on 15 October 2012
Gugerli
F. 1998.
Effect of elevation on sexual reproduction in alpine populations of Saxifraga oppositifolia (Saxifragaceae). Oecologia 114,
60–66.
Publisher Full Text
Hammer
Ø.,
Harper
D.A.T.
&
Ryan
P.D.
2001. PAST: paleontological statistics software package for education and data analysis. Palaeontologia Electronica 4, article no. 4.
Heiri
O.,
Lotter
A.F.
&
Lemcke
G. 2001.
Loss on ignition as a method for estimating organic and carbonate content in sediments: reproducibility and comparability of results. Journal of Paleolimnology 25,
101–110.
Publisher Full Text
Holderegger
R.
&
Abbott
R.J. 2003.
Phylogeography of the Arctic–alpine Saxifraga oppositifolia (Saxifragaceae) and some related taxa based on cpDNA and ITS sequence variation. American Journal of Botany 90,
931–936.
PubMed Abstract | Publisher Full Text
Holderegger
R.,
Stehlik
I.
&
Abbott
R.J. 2002.
Molecular analysis of the Pleistocene history of Saxifraga oppositifolia in the Alps. Molecular Ecology 11,
1409–1418.
PubMed Abstract | Publisher Full Text
Hultén
E.
&
Fries
M. (eds.) 1986. Atlas of North European vascular plants (north of the tropic of cancer). Vols. I-III. Königstein: Koeltz Scientific Books.
Husband
B.C.
&
Schemske
D.W. 2000.
Ecological mechanisms of reproductive isolation between diploid and tetraploid Chamerion angustifolium. Journal of Ecology 88,
689–701.
Publisher Full Text
Jørgensen
C.A.,
Sørensen
T.
&
Westergaard
M. 1958.
The flowering plants of Greenland. A taxonomical and cytological survey. Biologiske Skrifter 9,
1–172.
Kevan
P.G. 1972.
Insect pollination of High Arctic flowers. Journal of Ecology 60,
831–847.
Publisher Full Text
Klocke
N.L.
&
Fischbach
P.E. 1984. G84-690 estimating soil moisture by appearance and feel. Historical materials from University of Nebraska–Lincoln Extension. Paper 1201. Accessed on the internet at http://digitalcommons.unl.edu/extensionhist/ on 12 June 2012.
Müller
E.,
Eidesen
P.B.,
Ehrich
D.
&
Alsos
I.G. 2012.
Frequency of local, regional, and long-distance dispersal of diploid and tetraploid Saxifraga oppositifolia (Saxifragaceae) to Arctic glacier forelands. American Journal of Botany 99,
459–471.
Publisher Full Text
Oksanen
J. 2011. Multivariate analysis of ecological communities in R: vegan. Accessed on the internet at http://cc.oulu.fi/∼jarioksa/opetus/metodi/vegantutor.pdf on 5 May 2012.
Oksanen
J.,
Simpson
G.
&
Solymos
P. 2012.
Vegan: R functions for vegetation ecologists. Accessed on the internet at http://vegan.r-forge.r-project.org on 24 May 2012.
Parisod
C.,
Holderegger
R.
&
Brochmann
C. 2010.
Evolutionary consequences of autopolyploidy. New Phytologist 186,
5–17.
PubMed Abstract | Publisher Full Text
R Development Core Team 2011. R: a language and environment for statistical computing.
Vienna: R Foundation for Statistical Computing.
Rønning
O.I. 1996. The flora of Svalbard. Oslo: Norwegian Polar Institute.
Soltis
D.E.,
Soltis
P.S.,
Schemske
D.W.,
Hancock
J.F.,
Thompson
J.N.,
Husband
B.C.
&
Judd
W.S. 2007.
Autopolyploidy in angiosperms: have we grossly underestimated the number of species? Taxon 56,
13–30.
Soltis
D.E.,
Soltis
P.S.
&
Tate
J.A. 2004.
Advances in the study of polyploidy since plant speciation. New Phytologist 161,
173–191.
Publisher Full Text
Svendsen
J.I.,
Alexanderson
H.,
Astakhov
V.I.,
Demidov
I.,
Dowdeswell
J.A.,
Funder
S.,
Gataullin
V.,
Henriksen
M.,
Hjort
C.,
Houmark-Nielsen
M.,
Hubberten
H.W.,
Ingolfsson
O.,
Jakobsson
M.,
Kjaer
K.H.,
Larsen
E.,
Lokrantz
H.,
Lunkka
J.P.,
Lysa
A.,
Mangerud
J.,
Matiouchkov
A.,
Murray
A.,
Moller
P.,
Niessen
F.,
Nikolskaya
O.,
Polyak
L.,
Saarnisto
M.,
Siegert
C.,
Siegert
M.J.,
Spielhagen
R.F.
&
Stein
R. 2004.
Late quaternary ice sheet history of northern Eurasia. Quaternary Science Reviews 23,
1229–1271.
Publisher Full Text
Thompson
J.D.
&
Lumaret
R. 1992.
The evolutionary dynamics of polyploid plants: origins, establishment and persistence. Trends in Ecology & Evolution 7,
302–307.
Treier
U.A.,
Broennimann
O.,
Normand
S.,
Guisan
A.,
Schaffner
U.,
Steinger
T.
&
Müller-Schärer
H. 2009.
Shift in cytotype frequency and niche space in the invasive plant Centaurea maculosa. Ecology 90,
1366–1377.
PubMed Abstract | Publisher Full Text
Walker
D.A.,
Raynolds
M.K.,
Daniëls
F.J.A.,
Einarsson
E.,
Elvebakk
A.,
Gould
W.A.,
Katenin
A.E.,
Kholod
S.S.,
Markon
C.J.,
Melnikov
E.S.,
Moskalenko
N.G.,
Talbot
S.S.
&
Yurtsev
B.A. 2005.
The circumpolar Arctic vegetation map. Journal of Vegetation Science 16,
267–282.
Publisher Full Text
Winkler
M.,
Tribsch
A.,
Schneeweiss
G.M.,
Brodbeck
S.,
Gugerli
F.,
Holderegger
R.,
Abbott
R.J.
&
Schönswetter
P. 2012.
Tales of the unexpected: phylogeography of the Arctic–alpine model plant Saxifraga oppositifolia (Saxifragaceae) revisited. Molecular Ecology 21,
4618–4630.
PubMed Abstract | Publisher Full Text