RESEARCH/REVIEW ARTICLE
Tardigrades of Alaska: distribution patterns, diversity and species richness
Carl Johansson,1 William R. Miller,2 Eric T. Linder,3 Byron J. Adams4
& Erika Boreliz-Alvarado1
1 Department of Biology, Fresno City College, Fresno, CA 93741, USA
2 Department of Biology, Baker University, Baldwin City, KS 66006, USA
3 Department of Zoology, University of Wisconsin, Madison, WI 53706, USA
4 Department of Biology, Brigham Young University, Provo, UT 84602, USA
Abstract
During the summer of 2010, a biotic survey of tardigrades was conducted along a latitudinal transect in central Alaska from the Kenai Peninsula, via Fairbanks and the Arctic Circle to the coastal plain. Work was centred at the Toolik and Bonanza Creek Long Term Ecological Research Network sites and supplemented by opportunistic collections from the Kenai Peninsula and Anchorage areas. The 235 samples collected at 20 sites over 10 degrees of latitude yielded 1463 tardigrades representing two classes, three orders, 10 families, 23 genera and 73 species from 142 positive samples. A total of 50 species are new to Alaska, increasing the state's known species richness to 84. Several environmental metrics, such as pH, substrate, elevation, location and habitat were measured, recorded and analysed along the latitudinal gradient. Contrary to expectations, pH did not appear to be a predictor of tardigrade abundance or distribution. Density and species richness were relatively consistent across sites. However, the assemblages were highly variable within and between sites at only 14–20% similarity. We detected no correlation between species diversity and latitudinal or environmental gradients, though this may be affected by a high (59.9%) occurrence of single-species samples (containing individuals of only one species). Estimates of species richness were calculated for Alaska (118) and the Arctic (172). Our efforts increased the number of known species in Alaska to 84, and those results led us to question the validity of the estimate numbers.
Keywords
Tardigrade; Alaska; distribution; latitudinal gradient; pH; species richness.
Correspondence
Carl Johansson, Department of Biology, Fresno City College, Fresno, CA 93741, USA.
E-mail: birdbrain13@gmail.com
(Published: 22 May 2013)
Polar Research 2013. © 2013 C. Johansson et al. This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial 3.0 Unported License (http://creativecommons.org/licenses/by-nc/3.0/), permitting all non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Citation: Polar Research 2013, 32, 18793, http://dx.doi.org/10.3402/polar.v32i0.18793
Biodiversity is most often thought of as species richness or the number of species in a given area (Costello 2001), while declining biodiversity is a measure of species extinction (Chapin et al. 2000). The total number of species, the relationships between them and rates of extinction are particularly unclear (Jackson 2008). This is especially true for microscopic invertebrates in several under-studied taxonomic groups, such as the tardigrades (Costello et al. 2006).
Vicente (2010) used tardigrades and their significant survival capability as a case study to infer the danger of extinction for all other edaphic and hemi-edaphic micro-invertebrate taxa. He acknowledges that without baseline data sets, the impact(s) of global warming, habitat destruction and environmental pollution on micro-invertebrate diversity are difficult to measure. Clearly, there is a great need for biodiversity studies to support conservation of tardigrades and other minor taxonomic groups (Vicente 2010).
McInnes (1994) documented ca. 700 species of limno-terrestrial tardigrades, recorded more than half of those to the European Palearctic, but only 22 as cosmopolitan. Pilato & Binda (2001) considered 53 to be cosmopolitan and 493 Palearctic, but only 195 (39.5%) as Nearctic. Meyer & Hinton (2007) increased Nearctic numbers to 206 species. The current checklist (Guidetti & Bertolani 2005; Degma & Guidetti 2007; Degma et al. 2012) reports more than 1150 tardigrade species, of which ca. 170 are marine and over 1000 are limno-terrestrial species. Even so, the European (Palearctic) species bias does not make biological sense and is most likely explicable in terms of unequal sampling effort.
The first report of a tardigrade from Alaska was by Mathews (1938), who listed Pseudechiniscus suillus from south of Juneau. The second mention was 27 years later, when Schuster & Grigarick (1965) reported six species from Sand Point on the Alaskan Peninsula. Almost another 20 years passed before Dastych (1982) and Meininger & Spatt (1988) reported 27 and 12 species, respectively, from several sites along the Alaskan Highway, bringing the total to 32 (Table 1).
Table 1 Tardigrade collections in Alaska, 2009, arranged from north to south latitudes.
| Cluster code |
Site codea
|
Site |
Latitude |
Longitude |
Elevation (m) |
No. of samples |
No. of positive samples |
No. of specimens |
No. of species |
| Toolik Lake |
T |
Roadside Creek |
69.05930 |
148.49978 |
312.1 |
3 |
1 |
6 |
1 |
| Toolik Lake |
S |
Sagway River |
68.46167 |
148.50859 |
472.4 |
9 |
4 |
75 |
17 |
| Toolik Lake |
R |
Slope Mountain |
68.42592 |
149.02228 |
674.2 |
3 |
2 |
24 |
4 |
| Toolik Lake |
Q |
Kupa River |
68.38813 |
149.24832 |
755.9 |
5 |
1 |
1 |
1 |
| Toolik Lake |
P |
Toolik field station |
68.37695 |
149.35592 |
722.1 |
23 |
9 |
181 |
26 |
| Toolik Lake |
O |
Brooks Range |
68.14498 |
149.25371 |
906.8 |
14 |
3 |
25 |
9 |
| Arctic Circle |
N |
Arctic Circle |
66.33334 |
150.48644 |
361.8 |
1 |
1 |
3 |
1 |
| Arctic Circle |
M |
Finger Mountain |
66.21462 |
150.27686 |
650.7 |
12 |
7 |
92 |
18 |
| Bonanza Creek |
L |
Alyeska Pipeline |
64.92909 |
147.62944 |
238.7 |
3 |
1 |
6 |
1 |
| Bonanza Creek |
K |
Winner Creek |
64.51492 |
147.49820 |
205.7 |
13 |
6 |
11 |
3 |
| Bonanza Creek |
J |
Bonanza Creek |
64.42325 |
148.19439 |
160.6 |
31 |
22 |
176 |
20 |
| Kenai Peninsula |
I |
Crows Creek |
61.02707 |
149.07223 |
802.5 |
85 |
66 |
584 |
38 |
| Kenai Peninsula |
H |
Girdwood Outlook |
60.92840 |
149.34700 |
11.3 |
2 |
1 |
13 |
1 |
| Kenai Peninsula |
G |
Granite Mine |
60.57183 |
148.13301 |
183.2 |
5 |
3 |
89 |
5 |
| Kenai Peninsula |
F |
Kenai Lake |
60.49012 |
149.74716 |
247.2 |
1 |
1 |
5 |
1 |
| Kenai Peninsula |
E |
Moose Pass |
60.47070 |
149.40123 |
981.5 |
5 |
4 |
29 |
7 |
| Kenai Peninsula |
D |
Seward Highway |
60.39510 |
149.29020 |
375.2 |
9 |
3 |
29 |
5 |
| Kenai Peninsula |
C |
Swetman Mine |
60.36646 |
149.34685 |
165.5 |
3 |
1 |
25 |
2 |
| Kenai Peninsula |
B |
Lost Lake |
60.26337 |
149.42240 |
594.4 |
3 |
2 |
2 |
2 |
| Kenai Peninsula |
A |
Brown Bear Prospect |
60.20323 |
149.21058 |
177.7 |
5 |
4 |
87 |
13 |
| Total |
|
|
|
|
|
235 |
142 |
1463 |
|
| aSee Fig. 1. |
McInnes (1994) reports only four articles referencing Alaska, omitting Mathews (1938), but including Dastych (1991) for re-describing Isohypsibius sattleri (Richters, 1902), a species he had found in 1982. In her revision of the genus Minibiotus, Claxton (1998) reported the species Minibiotus intermedius from Denali National Park. Meyer & Hinton (2007) reviewed more than 100 publications relating to Nearctic species diversity realm, reporting 62 species from the “AS region”, which includes Alaska and northern Canada. They cite the above Alaskan species and articles, along with the 30 species reported from Axel Heiberg Island, Canada (79°N, 90°W) (Weglarska & Kuc 1980).
More recently, Schill, Forster, Dandekar & Wolf, 2010 described Paramacrobiotus fairbanksi from the Fairbanks area. We have added a new Oreella species (Calloway et al. 2011), bringing the total of known species in Alaska to 34, representing 53% of the Nearctic fauna (Meyer & Hinton 2007), but only 3% of global species richness.
We collected tardigrades along a north–south transect through central Alaska, comparing tardigrade communities in terms of sample similarity, density differences and composition in relation to pH and other environmental factors. We used our data to estimate tardigrade diversity for Alaska and the Arctic.
Materials, methods and study area
Study area
During the summer of 2010, a biotic survey of tardigrades was conducted along a latitudinal transect in central Alaska from the Kenai Peninsula, via Fairbanks and the Arctic Circle to the coastal plain. These were supplemented by additional collections gathered between 2007 and 2010 between −147° and −151° longitude (Fig. 1, Table 1).
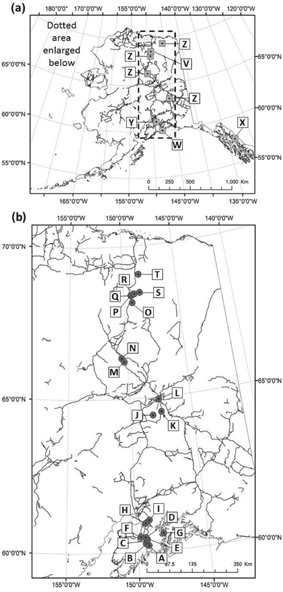
Fig. 1
Tardigrade collections: (a) prior collections by Meininger & Spratt (1988; V), Calloway et al. (2011; W), Mathews (1938; X), Schuster & Grigarick (1965; Y) and Dastych (1982; Z), and (b) collections in this study (letters correspond to site codes in Table 1).
Sampling sites were grouped into four latitudinal “regions”. (1) “Kenai” (60–61°N) comprised nine distinct sites between 60 and 62°N. (2) “Bonanza” (64°N) was made up of three sites in and around the Bonanza Creek Long Term Ecological Research site (LTER). (3) “Arctic Circle” (66°N) sampling took place on and near the Arctic Circle. (4) “Toolik” (68–69°N) samples were collected in the area of the Toolik LTER (Fig. 1, Table 1).
Sampling and processing
Hand-collected moss, lichen and leaf litter samples were placed in article bags labelled with global positioning system coordinates, substrate and habitat, and allowed to dry at ambient temperatures, prior to laboratory processing.
Dry samples were weighed on an auto-taring scale (Thermo Fisher Scientific, Waltham, MA) to±0.1 g and 10 ml of spring water (pH ca. 7.00) added per gram of sample. After 30 min, the sample was stirred, distressed and the pH taken via a LabPro pH sensor and Logger Pro data retrieving software (Vernier Software & Technology, Beaverton, OR). On the following day, all tardigrades were removed from the sample using an EMZ dissecting microscope (Meiji Techno America, Santa Clara, CA, USA), at 20–30×, reflected light and an Irwin loop. Tardigrade numbers were divided by sample dry weight to derive comparative density data.
Specimens were permanently mounted on slides in polyvinyl alcohol (PVA) media for identification (Miller 1997). Additional specimens were preserved for subsequent DNA analysis or scanning electron microscope imaging. All slides associated with this study are archived at the Academy of Natural Sciences in Philadelphia. Tissues samples are stored at −80°C at Brigham Young University, remnants of the habitat samples are currently stored at Fresno City College, and collection metadata are contained in an online database (http://tegrity.fresnocitycollege.edu/johansson/).
Tardigrades were identified using a BX 51differential interference contrast (DIC) microscope (Olympus, Center Valley, PA, USA) along with the keys of Pilato & Binda (2011), Nelson & McInnes (2002), Rammazzotti & Maucci (1983) and current literature. Mounted specimens were photographed via an Olympus DP72 high-resolution digital camera mounted on the DIC microscope. Classification is derived from Degma et al. (2012).
Statistical methods
To determine the potential influence of pH, we compared the pH of tardigrade-positive with tardigrade-negative samples using ANOVA to test for differences.
To determine the probability of finding positive tardigrade samples with the same frequency along our latitudinal gradient, we normalized the data via an arcsine transformation for a Pearson's correlation analysis of individual samples. This test was also applied in determining if species richness changed along the latitudinal gradient. ANOVA, using PROC GLM in the SAS software package (SAS Institute 2006), was used to test for differences in density and species richness with the defined clusters as our dependent variable. We used the Bray-Curtis similarity index metric and analysis of similarity (ANOSIM) order to identify how community composition changed between samples both within and between sites, applying a fourth-root transformation to reduce variance within the density data. We deemed this appropriate because so many of the samples consisted of only a few individuals, often representing a single species (Legendre & Legendre 1998).
Estimates of species numbers.
EstimateS version 8.2 software (Colwell et al. 2012) was used to estimate probable total tardigrade species numbers for central Alaska and the whole Arctic, reducing conflicts by eliminating genus-only identifications (Bartels & Nelson 2007). Data were divided into moss versus lichen habitats and high (>66°N) versus low (55–65°N) latitudes. Accumulation curves and estimated the species richness values were calculated for central Alaska based on the median of seven EstimateS estimators. Finally, the two habitat estimators were summed and percentage overlap (species occurring in both) was subtracted to estimate total species richness.
Results.
A total of 142 of the 235 (58.9%) samples taken from the 20 sites were positive for at least one tardigrade species. The 1463 individual tardigrades collected represent 2 classes, 3 orders, 5 super-families, 10 families, 23 genera and 73 species. Some 50 species are new to Alaska, bringing species richness to 84 (Table 2). Some 41 species were unique to particular regions, including low-latitude Kenai (23), high-latitude Toolik (10), mid–low latitude Bonanza and Arctic Circle (2). Furthermore, 40 (54.7%) species occurred at only one site and 12 (16.4%) in all four regions.
Table 2 Tardigrades of Alaska
| Family |
Genus |
Species |
1938a
|
1965a
|
1982a
|
1988a
|
1998a
|
2010a
|
2012a
|
Site codes (north to south)b
|
| Oreellidae |
|
|
|
|
|
|
|
|
| Oreella
|
|
|
|
|
|
|
|
|
|
|
|
|
O. chugachii Calloway, Miller, Johansson & Whiting, 2012 |
|
|
X |
K |
| Echiniscidae |
|
|
|
|
|
|
|
|
| Echiniscus
|
|
|
|
|
|
|
|
|
|
|
E. arctomys Ehrenberg, 1853 |
|
|
|
|
|
|
X |
C, G, I, M, P |
|
|
E. blumi blumi Richters, 1903 |
|
|
|
|
|
|
X |
M |
|
|
E. columinis Murray, 1911d
|
|
|
|
|
|
|
X |
G, I, K |
|
|
E. merokensis merokensis Richters. 1904 |
|
X |
|
|
|
X |
A |
|
|
E. oihonnae Richters, 1903 |
|
|
|
|
|
|
X |
J |
|
|
E. postojnensis Mihelcic, 1967c
|
|
|
|
|
|
|
X |
I |
|
|
E. quadrispinosus quadrispinosus Richters, 1902 |
|
|
|
|
X |
J, M |
|
|
E. robertsi Schuster & Grigarick, 1965 |
X |
|
|
|
|
|
|
|
|
E. testudo Doyère, 1840 |
|
|
|
|
| |
X |
I |
|
|
E. trisetosus Cuénot, 1932 |
|
|
|
|
| |
X |
R |
|
|
Echiniscus sp. A |
|
|
|
|
| |
X |
J |
|
|
Echiniscus sp. B |
|
|
|
|
|
|
X |
A, E, G, I, J, P, R, S |
| Hypechiniscus
|
|
|
H gladiator gladiator (Murray, 1905) |
X |
X |
|
|
|
X |
I, O |
| Pseudechiniscus
|
|
|
P. facettalis Petersen, 1951 |
|
|
|
|
|
|
X |
P |
|
|
P. suillus Ehrenberg, 1853 |
X |
X |
|
|
|
|
X |
I |
|
|
P. victor Ehrenberg, 1853 |
|
|
|
|
|
|
X |
S |
|
|
Pseudechiniscus sp. |
|
|
|
|
|
|
X |
I, P |
| Milnesiidae |
|
|
|
|
|
|
|
|
| Milnesium
|
|
|
|
|
|
|
|
|
|
|
M. eurystomum Maucci, 1991e
|
|
|
|
|
|
|
X |
I, M, P |
|
|
Milnesium sp. |
|
|
X |
|
| |
X |
I, J, M, O, P, S |
|
|
Milnesium n. sp. |
|
|
|
|
|
|
X |
J, O, S |
| Eohypsibiidae |
| Bertolanius (Amphibolus) |
|
|
Bertolanius sp. |
|
|
|
X |
|
|
|
|
| Calohypsibiidae |
| Calohypsibius
|
|
|
C. ornatus Richters, 1900 |
|
X |
|
|
|
|
X |
I, M |
| Hypsibiidae |
| Diphascon (Diphascon) |
|
|
D. alpinum Murray, 1906 |
|
X |
X |
|
|
|
X |
J |
|
|
D. bullatum Murray, 1905 |
|
|
X |
|
|
|
|
|
|
|
D. iltisi Schuster & Grigarick, 1965 |
|
|
X |
|
|
|
X |
P |
|
|
D. nodulosum Ramazzotti, 1957 |
|
|
|
|
|
|
X |
I |
|
|
D. oculatumoculatum Murray, 1906 |
|
|
|
|
|
|
X |
I, J, K, M, P |
|
|
D. oculatumalpinum Mihelcic, 1964 |
|
|
|
|
|
|
X |
I |
|
|
D. pingue pingue Marcus, 1936 |
|
|
|
X |
|
|
X |
I, J, P |
|
|
D. ramazzottii Robotii, 1970e
|
|
|
|
|
|
|
X |
O |
|
|
D. rugosum Bartoš, 1935 |
|
|
X |
|
|
|
|
|
|
|
D. stappersi Richters, 1911 |
|
|
|
|
|
|
X |
P |
|
|
Diphascon sp. |
|
|
|
|
|
|
X |
A, E, G, H, I, J, M, P |
| Diphascon (Adropion) |
|
|
D. arduifrons Thulin, 1928 |
|
|
|
|
|
|
X |
I, P |
|
|
D. belgicae Richters, 1911 |
|
|
|
|
|
|
X |
I, P |
|
|
D. prosirostre Thulin, 1928 |
|
|
X |
X |
|
|
X |
A |
|
|
D. scoticum Murray, 1905 |
|
|
X |
X |
|
|
X |
I |
| Hypsibius
|
|
|
H. convergens Urbanowicz, 1925 |
|
|
X |
|
|
|
X |
M, Q |
|
|
H. dujardini Doyère, 1840 |
|
|
|
X |
|
|
X |
J |
|
|
H. pallidus Thulin, 1911 |
|
|
X |
X |
|
|
X |
C, D, I, J, L, O, P, S |
|
|
Hypsibius sp. |
|
|
|
|
|
|
|
B, F, G, P |
| Mesocrista
|
|
|
M. marcusi Rudescu, 1964c
|
|
|
|
|
|
|
X |
I, P |
|
|
M. spitzbergensis (Richters, 1903) |
|
|
X |
X |
|
|
|
|
| Platicrista
|
|
|
P. angustata Murray, 1905 |
|
|
X |
X |
|
|
X |
D, J, K, M |
|
|
P. cheleusis Kathman, 1990 |
|
|
|
|
|
|
X |
I |
|
|
P. itaquasconoide Durante Pasa & Maucci, 1975c
|
|
|
|
X |
I |
| Ramazzottidae |
| Ramazzottius
|
|
|
R. oberhaeuseri Doyère, 1840 |
|
|
X |
|
|
|
X |
B, I, J, M, R |
|
|
Ramazzottius sp. |
|
|
|
|
|
|
X |
I, J |
| Isohypsibioiidae |
| Isohypsibius
|
|
|
I. brevispinosus Iharos, 1966e
|
|
|
|
|
|
|
X |
I |
|
|
I. duranteae Maucci, 1964c
|
|
|
|
|
|
|
X |
S |
|
|
I. pauper Mihelcic, 1971c
|
|
|
|
|
|
|
X |
S |
|
|
I. prosostomus Thulin, 1928 |
|
|
|
|
|
|
X |
A, I, S |
|
|
I. sattleri Richters, 1902 |
|
|
X |
|
|
|
X |
J |
|
|
I. tuberculatus (Plate, 1888) |
|
|
X |
|
|
|
|
|
|
|
Isohypsibius sp. A |
|
|
X |
|
|
|
X |
J |
|
|
Isohypsibius sp. B |
|
|
|
|
|
|
X |
E, I, M, P, S |
| Pseudobiotus
|
|
|
|
|
|
|
|
|
|
|
Pseudobiotus sp. |
|
|
|
|
|
X |
S, T |
| Macrobiotidae |
| Adorybiotus
|
|
|
A. granulatus (Richters, 1903) |
|
|
X |
X |
|
|
X |
L, M, Q |
|
|
Adorybiotussp. |
|
|
|
|
|
|
X |
M |
| Macrobiotus
|
|
|
M. australis Pilato & D'Urso, 1976c
|
|
|
|
|
|
X |
Q |
|
|
M. crenulatus Richters, 1904e
|
|
|
|
|
|
|
X |
I, K, P |
|
|
M. echinogenitus Richters, 1904 |
|
|
X |
|
|
|
|
|
|
|
M. furciger Murray, 1907d
|
|
|
|
|
|
|
X |
X |
|
|
M. grandis Richters, 1911 |
|
|
|
|
|
|
X |
A |
|
|
M. harmsworthi Murray, 1907 |
|
|
X |
X |
|
|
X |
H, K, L, M, O, Q, R |
|
|
M. hufelandi C.A.S. Schultze, 1833 |
|
X |
X |
X |
|
|
X |
A, C, F, H, I, K, L, M, O, Q, R |
|
|
M. islandicus Richters, 1904 |
|
X |
X |
|
|
|
X |
K |
|
|
M. montanus Murray, 1910 |
|
|
X |
|
|
|
|
|
|
|
M. occidentalis Murray, 1910 |
|
|
|
|
|
|
X |
K, N, Q |
|
|
M. pallarii Maucci, 1954 |
|
|
|
|
|
|
X |
H |
|
|
M. recens Cuénot, 1932 |
|
|
|
|
|
|
X |
H |
|
|
M. spectabilis Thulin, 1928 |
|
|
X |
|
|
|
X |
A, I, N |
|
|
Macrobiotus sp. |
|
|
|
|
|
|
X |
X |
| Minibiotus
|
|
|
M. intermedius Plate, 1888 |
|
|
X |
|
X |
|
X |
H, L, M, Q |
|
|
Minibiotus sp. |
|
|
|
|
|
|
X |
A, I, Q |
| Paramacrobiotus
|
|
|
P. areolatus Murray, 1907 |
|
|
|
|
|
|
X |
A, M |
| Richtersius
|
|
|
R. coronifer Richters, 1903e
|
|
|
|
|
|
|
X |
I, J, L, Q |
| Tenuibiotus
|
|
|
T. fairbanksi Schill, Forster, Dandekar & Wolf, 2010 |
|
|
X |
|
|
|
|
T. hystricogenitus Maucci, 1988c
|
|
|
|
|
|
|
X |
Q |
|
|
T. willardi Pilato, 1977 |
|
|
X |
|
|
|
|
|
| Murrayidae |
|
|
|
|
|
|
|
|
| Dactylobiotus
|
|
|
D. ambiguus (Murray, 1907)e
|
|
|
|
|
|
|
X |
Q |
| Murrayon
|
|
|
M. hastatus Murray, 1907 |
|
|
|
|
|
|
X |
T |
|
|
M. hibernicus Murray, 1911 |
|
|
X |
|
|
|
|
|
|
|
Totals |
1 |
7 |
27 |
11 |
1 |
1 |
73 |
Total species 84 |
| a1938 is Mathews 1938; 1965 is Schuster & Grigarick 1965; 1982 is Dastych 1982; 1988 is Meininger & Spatt 1988; 1998 is Claxton; 2010 is Schill et al.; and 2012 is this study. |
| bSee Table 1 for site codes. |
| cNew records for North America. |
| dNew record for the Americas. |
| eNew records for the United States. |
There were no significant pH differences between samples that contained tardigrades and those that did not (d.f.1,146F=0.177, P=0.675). Correlation analysis indicated no significant (coefficient 0.189, P=0.365) relationship between the probability of a positive sample and latitude. Indeed positive sample probability was relatively consistent throughout our study sites. There was no relationship between species richness and latitude (Pearson's correlation coefficient = − 0.053, P=0.600).
ANOVA indicated that tardigrade density did not vary significantly across each cluster d.f.3,96F=0.338, P=0.798), while Tukey's Honestly Significant Difference (HSD) test showed none of the clusters to be significantly different from each another (Fig. 2) suggesting a relatively constant tardigrade density across the latitudinal gradient.
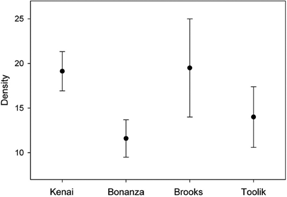
Fig. 2
Density of tardigrades from our four clusters of sites. No significant differences were found.
However, ANOVA did show that species richness varies between clusters (d.f.3,96F=2.63, P=0.055), while Tukey's HSD test indicated the only significant pairwise difference was (high) Anchorage compared to (low) Bonanza (Fig. 3). ANOSIM suggests sample similarity within samples is very low, averaging 14–19% (Table 3), but is significantly dissimilar between clusters, irrespective of distance between samples (Global R=0.037, P=0.093). Further examination of our data, using both principle component analysis and cluster analysis strongly supported our previous results.
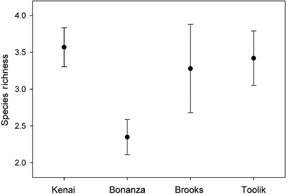
Fig. 3
Tardigrade richness from our four clusters of sites. Only the Bonanza and Kenai sites were significantly different from one another.
Table 3 Similarity (%) within and among clusters for tardigrade communities across Alaska.
| |
Kenai Peninsula |
Bonanza |
Brooks Range |
Toolik |
| Kenai Peninsula |
17.76 |
16.42 |
20.20 |
13.96 |
| Bonanza |
|
18.92 |
18.55 |
14.34 |
| Brooks Range |
|
|
18.99 |
15.49 |
| Toolik |
|
|
|
14.08 |
EstimateS estimators proposed an Alaskan species richness of between 90 and 160 species; the median (Jack1 for both habitats) of all estimators estimates total species richness at 118 species or ca. 10% of global diversity.
Discussion
Taxonomic notes
Echiniscus arctomys sensu stricto, Diphascon (Diphascon) rugosum sensu stricto and Tenuibiotus willardi sensu stricto were previously reported from Alaska as “cf.” or uncertain identifications. Mechalczyk et al. (2012) recently re-described Milnesium tardigradum from European material and stated that the presence of the species outside Europe was unconfirmed. Thus, all previous records from North America must be considered Milnesium sp. and we have found no Milnesium tardigradum (sensu Mechalczyk et al. 2012) in our Alaskan collections, but we are confident these contain at least one new species to be described later.
Ecological notes.
While the survey was expected to add to the known tardigrade diversity of Alaska, a 150% increase is remarkable, as was the large number of species that occurred at only one sampling site. While we had collected large numbers of Oreella chugachii at one site, repeated sampling, over three years, in close proximity (within 500 m) yielded no other O. chugachii populations despite consistent finds at the original site. This unusual distributional pattern leads us to question species number estimates. It is unlikely that over 70% (84 of 118) of the predicted number of species in Alaska have been captured with this limited sampling scheme. Given Alaska's great size and apparent abundance of suitable habitat, we are convinced we could not be close to capturing all the extant species.
Although pH is often cited as an important factor influencing the distribution (e.g., site suitability), density and species richness of tardigrades (Meininger et al. 1985; Hoffman 1987; Dastych 1988; Meininger & Spatt 1988; Mitchell et al. 2009), not all studies have shown a strong influence of pH on species distributions (Johansson et al. 2011). This study does not support the influence of pH on either the probability of samples containing tardigrades nor on species richness. Certainly, upper and lower pH limits reduce distribution of tardigrades and those ranges may vary by species, but in our study, the presence or absence of tardigrades in microhabitats within a suitable range of pH appears to be either random or controlled by other unmeasured variables.
These observations are consistent with the idea that the tardigrade communities along the study transect may be structured as much by stochastic factors associated with dispersal as they are by variables associated with habitat suitability (Convey & McInnes 2005). For example, while pH may be important in influencing site suitability, it fails to emerge as “significant” because so many sites (including pH-suitable ones) remain unoccupied.
These results suggest minimal differences between the structure of tardigrade communities across Alaska with respect to species richness and density. The variation was insufficient to produce any significant patterns. Many taxonomic groups exhibit latitudinal diversity gradients (Rosenzweig 1995; Wu et al. 2009; Wu et al. 2011), but it does not appear to hold for tardigrades in this particular study area, suggesting the distribution data are not complete, or at least representative. It is likely the geographic distributions of many species are underestimated because Artic species diversity is grossly underestimated.
Interestingly, within and among site, similarity indices were both within a relatively narrow range (14–20%) indicating that samples in close proximity to one another were no more likely to share a similar tardigrade assemblage as any other two random locations across Alaska (Table 3). The low similarity in Alaskan tardigrade communities is largely the result of a few cosmopolitan species and a large number of species that appear in single/very few samples. Clearly, more work needs to be done to determine if, and at what spatial and temporal scales, common distributional patterns hold for these microscopic animals.
Meyer & Hinton (2007) reported that the Arctic and sub-Arctic tardigrade fauna was taxonomically quite different than that of the remaining North America. Our results tend to corroborate that observation. Comparison of the species make-up of other large-scale surveys in North America show that our Alaskan species list has only 14% overlap (19 out of 139 species occurred in both studies) with the Great Smoky Mountain National Park (GSMNP; Bartels & Nelson 2007), and a 21% overlap (21 out of 102 species) with the western North American study done by Schuster & Grigarick (1965).
Bartels & Nelson (2007) examined 6220 specimens from 231 samples in GSMNP and developed a list of 73 species. Using EstimateS version 7.5, they estimated the probable species richness in the park to be 96 with a range of 86–105 species. Following their methods, we estimated the probable species richness for central Alaska to be 118, within a range of 90–160 species. Considering that the GSMNP is located at temperate latitudes and is 0.13% of the land mass of Alaska, these are not directly comparable studies. However, the model they established is informative as to the prediction of the diversity of a defined area.
The new list (Table 2) of 84 species from central Alaska (55–69N, 149°W) surpasses the results of the other major Arctic collections. Peterson (1951) reported 34 species from sites around Greenland (62–82°N, 40°W). Weglarska & Kuc (1980) reported 63 species from Axel Heiberg Island, Canada (79°N, 90°W). Dastych (1985) reported 48 species from Spitsbergen (79°N, 19°E). Biserov (1999) reported 59 species from the Novaya Zemlya (74°N, 56°E). Biserov (1996) also reported 71 species from the Taimyr Peninsula (74°N, 96°E) and finally, Biserov (1998) reported 42 species from the Pacific islands of Komandorskyie (55°N, 167°E). Combined, these reports identify more than 127 species from north of 55°N, of which 58 (46%) were reported from only a single area and only nine species (7%) occurred in all Arctic areas. After combining these reports with the Alaskan collections, our EstimateS 8.2 estimators predicted that a total of 170 species (range 132–195) are likely to occur in the whole Arctic. Further whole-Arctic analysis is beyond the scope of this study.
To summarize, previous studies have only documented a small portion of the Nearctic tardigrade community. While general ecological patterns may apply to this group of meiofauna, the spatial scale at which this study was conducted failed to identify any traditional distributional patterns. The relatively large number of species that occurred at a single site may also have obscured patterns. Identification of which environmental parameters, if any, strongly influence the distribution of tardigrades remains elusive. We feel that additional data of the spatial–temporal variation in abundance of tardigrades and the processes that generate them are needed to understand the basic ecology of this under-studied group.
Acknowledgements
This project was supported by US National Science Foundation grants, numbers DEB 0640847 to Baker University, DEB 0641051 to Fresno City College and DEB0640959 to Brigham Young University. We are grateful for the geographic information system-based Alaska map produced by Dr Jeff Miller of Missoula, MT. Stephanie Calloway, Carleigh Takemoto, Alex Arnett and Brittany Deegan provided hundreds of hours of invaluable effort in sampling, identification and database management. Philip Pugh was gracious with his time, independently verifying and confirming our findings using additional statistical tests. Bill Johansson provided suggestions and motivation to improve and shape the manuscript. We also want to particularly thank the reviewers of this manuscript. They took an exceptional amount of time and expended considerable effort in reviewing, editing and improving this article. Their efforts are greatly appreciated by the authors.
References
Bartels
P.
&
Nelson
D.R. 2007.
An evaluation of species richness estimators for tardigrades of the Great Smoky Mountains National Park, Tennessee and North Carolina, USA. Proceedings of the Tenth International Symposium on Tardigrada. Journal of Limnology 66 (Supp 1),
104–110.
Biserov
V. 1996.
Tardigrades of the Taimyr Peninsula with descriptions of two new species. Zoological Journal of the Linnean Society 116,
215–237.
Publisher Full Text
Biserov
V. 1998.
The Tardigrada of the Komandorskiye Islands, with a description of Dactylobiotusdervizi sp. n. (Eutardigrada, Macrobiotidae). Entomologische Mitteilungenausdem Zoologischen Museum Hamburg 12,
327–336.
Biserov
V. 1999.
A review of the Tardigrada from Novaya Zemlya, with descriptions of three new species, and an evaluation of the environment in this region. Zoologischer Anzeiger 238,
169–182.
Calloway
S.,
Miller
W.R.,
Johansson
C.
&
Whiting
J. 2011.
Tardigrades of North America: Oreella chugachii nov. sp. (Eutardigrada, Echiniscoide, Oreellidae) a new species from Alaska. Proceedings of the Biological Society of Washington 124,
28–39.
Publisher Full Text
Chapin
F.S.,
Zavaleta
E.S.,
Eviner
V.T.,
Naylor
R.I.,
Vitousek
P.M.,
Reynolds
H.L.,
Hooper
D.U.,
Lavorel
S.,
Sala
O.E.,
Hobble
S.E.,
Mack
M.C.
&
Diaz
S. 2000.
Consequences of changing biodiversity. Nature 405,
234–242.
Publisher Full Text
Claxton
S. 1998.
A revision of the genus Minibiotus (Tardigrada: Macrobiotidae) with descriptions of eleven new species from Australia. Records of the Australian Museum 50,
125–160.
Publisher Full Text
Colwell
R.K.,
Chao
A.,
Gotelli
N.J.,
Lin
S.Y.,
Mao
C.X.,
Chazdon
R.L.
&
Longino
J.T. 2012.
Models and estimators linking individual-based and sample-based rarefaction, extrapolation and comparison of assemblages. Journal of Plant Ecology 5,
3–21.
Publisher Full Text
Convey
P.
&
McInnes
S.J. 2005.
Exceptional tardigrade-dominated ecosystems in Ellsworth Land, Antarctica. Ecology 86,
19–27.
Publisher Full Text
Costello
M.J. 2001.
To know, research, manage, and conserve marine biodiversity. Océanis 24,
25–49.
Costello
M.J.,
Emblow
C.S.,
Bouchet
P.
&
Legakis
A. 2006.
European marine biodiversity inventory and taxonomic resources: state of the art and gaps in knowledge. Marine Ecology Progress Series 316,
257–268.
Publisher Full Text
Dastych
H. 1982.
An annotated list of Alaskan Tardigrada. Polish Polar Research 3,
95–102.
Dastych
H. 1985.
West Spitsbergen Tardigrada. Acta Zoologica Cracoviensia 28,
169–214.
Dastych
H.
1988. The Tardigrada of Poland. Monografie Fauny Polski 16. Warsaw: Polska Akademia Nauk.
Dastych
H. 1991. Isohypsibius sattleri (Richters, 1902), a valid species (Tardigrada). Senckenbergiana Biologica 71,
181–189.
Degma
P.,
Bertolani
R.
&
Guidetti
R. 2012. Actual checklist of Tardigrada species (2009–2012, Ver. 21: 30-06-2012). Accessed on the internet at http://www.tardigrada.modena.unimo.it/miscellanea/Actual%20checklist%20of%20Tardigrada.pdf on 7 July 2012.
Degma
P.
&
Guidetti
R. 2007.
Notes to the current checklist of Tardigrada. Zootaxa 1579,
41–53.
Guidetti
R.
&
Bertolani
R. 2005.
Tardigrade taxonomy: an updated checklist of the taxa and a list of characters for their identification. Zootaxa 845,
1–46.
Hoffman
I. 1987. Habitat preference of the most frequent moss-living Tardigrada in the area of Giessen (Hessen). In
R.
Bertolani
(ed.): Biology of tardigrades. Selected symposia and monographs U.Z.I. 1. Pp. 211–216.
Modena: Mucchi.
Jackson
J.B.C. 2008.
Ecological extinction and evolution in the brave new ocean. Proceedings of the National Academy of Science of the United States of America 105,
11458–11465.
Publisher Full Text
Johansson
C.,
Calloway
S.,
Miller
W.R.
&
Linder
E.T. 2011.
Urban and rural tardigrade communities: are they distinct? Is pH a determinant? A case study from Fresno County, California. Pan-Pacific Entomologist 87,
88–99.
Publisher Full Text
Mathews
G.B. 1938.
Tardigrada from North America. American Midland Naturalist 19,
619–627.
Publisher Full Text
McInnes
S.J. 1994.
Zoogeographic distribution of terrestrial/freshwater tardigrades from current literature. Journal of Natural History 28,
257–352.
Publisher Full Text
Mechalczyk
L.,
Welnicz
W.,
Frohme
M.
&
Kaczmarek
L. 2012.
Redescriptions of three Milnesium Doyère, 1840 taxa (Tardigrada: Eutardigrada: Milnesiidae), including the nominal species for the genus. Zootaxa 3154,
1–20.
Meininger
C.A.
&
Spatt
P.D. 1988.
Variations of tardigrade assemblages in dust impacted Arctic mosses. Arctic and Alpine Research 20,
24–30.
Publisher Full Text
Meininger
C.A,
Uetz
G.W.
&
Snider
J.A. 1985.
Variation in epiphytic microcommunities (tardigrade–lichen–bryophyte assemblages) of the Cincinnati, Ohio area. Urban Ecology 9,
45–61.
Publisher Full Text
Meyer
H.A.
&
Hinton
J.G. 2007.
Limno-terrestrial Tardigrada of the Nearctic realm. Proceedings of the Tenth International Symposium on Tardigrada. Journal of Limnology 66 (Supp 1),
97–103.
Miller
W.R. 1997.
Tardigrades: bears of the moss. Kansas School Naturalist 43,
1–16.
Mitchell
C.,
Miller
W.R.
&
Davis
B. 2009.
Tardigrades of North America: influence of substrate on habitat selection. Journal of the Pennsylvania Academy of Science 83,
10–16.
Nelson
D.R.
&
McInnes
S.J. 2002. Tardigrada. In
S.D.
Rundle
et al.
(eds.): Freshwater meiofauna: biology and ecology. Pp. 177–215.
Leiden: Backhuys Publishers.
Petersen
B.
1951. The tardigrade fauna of Greenland: a faunistic study with some few ecological remarks. Meddelelser om Grønland 150. Copenhagen: Commission for Scientific Investigations in Greenland.
Pilato
G.
&
Binda
M.G. 2001.
Biogeography and limno-terrestrial tardigrades: are they truly incompatible binomials? Zoologischer Anzeiger 240,
511–516.
Publisher Full Text
Pilato
G.
&
Binda
M.G. 2011.
Definitions of the families and subfamilies, genera and subgenera of the Eutardigrada, and keys to their identification. Zootaxa 2404,
1–54.
Rammazzotti
G.
&
Maucci
W. 1983. Il phylum Tardigrada. III edizione riveduta e aggiornata. (The phylum Tardigrada. Third edn., revised and updated.) Memorie dell'Istituto Italiano di Idrobiologia 41.
Verbiana-Pallanza: Institute of Ecosystem Study.
Rosenzweig
M.L.
1995. Species diversity in space and time. Cambridge: Cambridge University Press.
SAS Institute 2006. Statistics, version 9.1.
Cary,
NC: SAS Institute.
Schuster
R.O.
&
Grigarick
A.A. 1965.
Tardigrada from western North America: with emphasis on the fauna of California. University of California Publications in Zoology 76,
1–67.
Vicente
F. 2010.
Micro-invertebrate conservation: forgotten biodiversity. Biodiversity Conservation 19,
329–3634.
Publisher Full Text
Weglarska
B.
&
Kuc
M. 1980.
Heterotardigrada from Axel Heiberg Island. Zeszyty Naukowe Uniwersytetu Jagiellonskiego. Prace Zoologiczne 26,
53–66.
Wu
T.H.,
Ayres
E.,
Bardgett
R.D.,
Wall
D.H.
&
Garey
J.R. 2011.
Molecular study of worldwide distribution and diversity of soil animals. Proceedings of the National Academy of Sciences of the United States of America 108,
17720–17725.
Publisher Full Text
Wu
T.H.,
Ayres
E.,
Li
G.,
Bardgett
R.D.,
Wall
D.H.
&
Garey
J.R. 2009.
Molecular profiling of soil animal diversity in natural ecosystems: incongruence of molecular and morphological results. Soil Biology & Biochemistry 41,
849–857.
Publisher Full Text