RESEARCH/REVIEW ARTICLE
Abundance and breeding distribution of seabirds in the northern part of the Danco Coast, Antarctic Peninsula
Diego González-Zevallos,1 M. Mercedes Santos,2 Emilce F. Rombolá,2 Mariana A. Juáres2,3 & Néstor R. Coria2
1 Centro Nacional Patagónico (Consejo Nacional de Investigaciones Científicas y Técnicas), Blvd. Brown 2915 (9120), Puerto Madryn, Chubut, Argentina
2 Instituto Antártico Argentino – División Biología, Cerrito 1248 (1010), Buenos Aires, Argentina
3 Consejo Nacional de Investigaciones Científicas y Técnicas, Rivadavia 1917 (1033), Buenos Aires, Argentina
Abstract
Seabird abundances and breeding distribution have the potential to serve as ecological indicators. The western Antarctic Peninsula is one of the three sites in the world with the greatest increases in local temperature during the last 50 years. The aim of this study was to monitor the distribution and abundance of breeding populations of seabirds in the northern sector of the Danco Coast, north-west of the Antarctic Peninsula, during the breeding season 2010/11. The birds were the Wilson′s storm petrel (Oceanites oceanicus), South Polar skua (Stercorarius maccormicki), kelp gull (Larus dominicanus), Antarctic tern (Sterna vittata), snowy sheathbill (Chionis alba), chinstrap penguin (Pygoscelis antarctica), southern giant petrel (Macronectes giganteus), gentoo penguin (Pygoscelis papua), Cape petrel (Daption capense) and Antarctic shag (Phalacrocorax bransfieldensis). Annual breeding population growth increased in pygoscelids, southern giant petrel and sheathbill, and for the remaining species, breeding population trends were stable. Given that seabird populations can provide valuable information on the conditions of their feeding and nesting environments, this study highlights the need to maintain basics monitoring studies.
Keywords
Seabird abundances; breeding distribution; Danco Coast; Antarctic Peninsula.
Correspondence
Diego González-Zevallos, Centro Nacional Patagónico, Blvd. Brown 2915 (9120), Puerto Madryn, Chubut, Argentina.
E-mail: diegue@cenpat.edu.ar
(Published: 28 February 2013)
Polar Research 2013. © 2013 D. González-Zevallos et al. This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial 3.0 Unported License (http://creativecommons.org/licenses/by-nc/3.0/), permitting all non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Citation: Polar Research 2013, 32, 11133, http://dx.doi.org/10.3402/polar.v32i0.11133
Studies of seabird populations can provide valuable indicators on the conditions of their feeding and nesting environments in relation to global-scale processes (Croxall et al. 1988). Climatic and oceanographic variability and change have been shown to affect seabirds, often with profound consequences, like reduced breeding success and altered breeding cycles for some species (Chambers et al. 2011). Three regions in the world are recognized as having warmed more rapidly during the last 50 years (Vaughan et al. 2003). Located in the Southern Hemisphere, one of these three regions includes the region west of the Antarctic Peninsula (Smith et al. 1999; Vaughan et al. 2001; Gille 2002; Cook et al. 2005). The populations of various species of penguins in this region have shown responses to increases in air temperature, retreat of the glaciers and decreases in the frequency of cold years in association with a decrease of sea ice in winter and its negative effects on the abundance of krill (Siegel & Loeb 1995; Loeb et al. 1997; Fraser & Hofmann 2003). In this context, long-term surveys are essentials to document population trends.
The present study evaluated the breeding population size and distribution of marine birds in the northern sector of the Danco Coast, Antarctic Peninsula, including the Antarctic Specially Protected Area (ASPA) No. 134.
Study area and methods
The study was conducted in the northern sector of the Danco Coast during the 2010/11 breeding season, which lasts from 24 December 2010 until 20 February 2011. Several sites were surveyed between Cape Herschell and Spring Point (Fig. 1). The sampling area covered the Mar Rock, Cierva Point, Moss Island, Midas Island and Sterneck Islands, all lying within ASPA No. 134. Counts of apparently occupied nests of penguins were made by eye from the single highest point of each selected area (exposed rocky patches), with contiguous areas covering each colony in its entirety. Counts of apparently occupied nest of flying birds were made by eye and by using 40× binoculars. We obtained the number of apparently occupied nests in each area based on the mean of the three counts (Table 1). Counts of Wilson′s storm petrels (Oceanites oceanicus) were also made but were not considered due to their high inaccuracy. The presence of two possible nests of brown skua (Stercorarius lonnbergi) was noted in Cierva Point, but for this study we considered all these birds as South Polar skuas (Stercorarius maccormicki). Changes in breeding population sizes were compared with those obtained in 1997/98 (Favero et al. 2000) for the same sampling localities. The annual breeding population growth rate was calculated as λ=(Ns2/Ns1)^(1/T), where N represents the breeding population size and s the season (s1: 1997/98 and s2: 2010/11) and T is the number of seasons between the two surveys (T=13). Lambda (λ) indicates if the population increases (λ>1), decreases (λ<1) or remains stable (λ=1) through time (Caswell 1989).
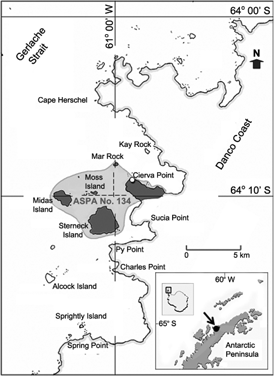
Fig. 1
Map of the study area showing the different localities sampled and the Primavera Base (marked with an open circle) and the Antarctic Specially Protected Area (ASPA) No. 134 (light grey), including the marine areas between the islands and the mainland.
Table 1 Positions and dates of population surveys conducted at each site from December 2010 to February 2011.
| Locality |
Latitude |
Longitude |
Date |
| Cierva Point |
64°09′28.0′′S |
60°57′14.5′′W |
30 Dec. |
| Moss Islands |
64°09′56.3′′S |
61°02′05.7′′W |
8 Jan. |
| Sucia Point |
64°10′50.3′′S |
60°57′13.0′′W |
10 Jan. |
| Py Point |
64°13′25.4′′S |
61°00′13.0′′W |
11 Jan. |
| Sterneck Island |
64°12′03.7′′S |
60°59′12.7′′W |
12 Jan. |
| Mar Rock |
64°08′35.3′′S |
60°59′06.6′′W |
15 Jan. |
| Kay Rock |
64°07′33.8′′S |
60°55′51.6′′W |
17 Jan. |
| Charles Point |
64°14′04.3′′S |
60°59′40.1′′W |
20 Jan. |
| Midas Island |
64°10′18.3′′S |
61°05′05.9′′W |
6 Feb. |
| Alcock Island |
64°14′13.9′′S |
61°07′21.3′′W |
9 Feb. |
| Sprightly Island |
64°16′58.5′′S |
61°04′34.3′′W |
9 Feb. |
| Spring Point |
64°17′38.5′′S |
61°03′00.5′′W |
9 Feb. |
| Cape Herschel |
64°04′40.7′′S |
61°01′53.9′′W |
12 Feb. |
Results
The survey registered the presence of a total of 11 species of seabirds, 10 of them breeding in the study area. We recorded the presence of snow petrel (Pagodroma nivea) on two visits to Moss Island. Although no nests were found, we suspect that this highly cryptic species breeds on the island. The species seen to be breeding were, from most common to least common: Wilson′s storm petrel, South Polar skua, kelp gull (Larus dominicanus), Antarctic tern (Sterna vittata), snowy sheathbill (Chionis alba), chinstrap penguin (Pygoscelis antarctica), southern giant petrel (Macronectes giganteus), gentoo penguin (Pygoscelis papua), Cape petrel (Daption capense) and Antarctic shag (Phalacrocorax bransfieldensis). Sterneck Island was the site with the highest number of breeding species (nine species), followed by Py and Alcock and Midas islands (eight species) and then by Moss Islands and Cierva Point (seven species). No other site exceeded six species of seabirds present.
In pygoscelids, gentoo penguin breeding colonies showed increases in all localities analysed (Table 2). The breeding colonies of chinstrap penguin showed increases and decreases, but the overall trend for the study area was an increase (Table 3). In flying birds the annual population growth rates showed increases in southern giant petrel and sheathbill and decreases in Cape petrel and kelp gull. For the remaining flying birds, breeding population trends were stable (Table 4).
Table 2 Number of breeding pairs per location for gentoo penguin in the northern sector of Danco Coast. Parentheses indicate the year the count was conducted. Lambda (λ) represents the annual breeding population growth rate, N represent the breeding population size and s the season (s1: 1997/98 and s2: 2010/11).
| Locality |
Novatti (1978)
|
Poncet & Poncet (1987)
|
Quintana et al. (1998)
|
Favero et al. (2000) (Ns1)
|
This study (Ns2) |
λ
|
| Cierva Point |
559–614 (1954–58) |
600 (1984) |
800–1041 (1991–96) |
593 (1998) |
2680 |
1.12 |
| Sterneck Island |
|
450 (1987) |
|
905(1998) |
2795 |
1.09 |
| Py Point |
|
130 (1987)a
|
|
390 (1998)a
|
655 |
1.06 |
| Charles Point |
|
|
|
|
140 |
|
| Total |
– |
1180 |
– |
1888 |
6270 |
1.10 |
| aLocalities grouped. |
Table 3 Number of breeding pairs by location for chinstrap penguin in the northern sector of Danco Coast. Parentheses indicate the year the count was conducted. Lambda (λ) represents the annual breeding population growth rate, N represent the breeding population size and s the season (s1: 1997/98 and s2: 2010/11).
| Locality |
Muller-Schwarze (1975)
|
Poncet & Poncet (1987)
|
Woehler (1993)
|
Favero et al. (2000) (Ns1)
|
This study (Ns2) |
λ
|
| Cape Herschel |
|
|
|
316 (1998) |
650 |
1.06 |
| Kay Rock |
|
|
|
21 (1998) |
0 |
0.00 |
| Mar Rock |
|
500 (1984) |
|
1553 (1998) |
2763 |
1.05 |
| Midas Island |
2060 (1971) |
200 (1987) |
|
546 (1998) |
180b
|
0.92 |
| Sterneck Island |
|
1100 (1987) |
|
152b (1998) |
33 |
0.89 |
| Py Point |
|
10 (1987)a
|
|
13 (1998)a
|
10 |
0.98 |
| Charles Point |
|
|
|
|
0 |
|
| Alcock Island |
7710 (1971) |
10000 (1971) |
3000 (1990) |
605 (1998) |
1100 |
1.05 |
| Spring Point |
|
85 (1984) |
60 (1990) |
180 (1998)a
|
0 |
0.96 |
| Sprightly Island |
|
|
|
|
110 |
|
| TOTAL |
9770 |
11895 |
3060 |
3386 |
4846 |
1.03 |
| aLocalities grouped. |
| bPossible underestimation. |
Table 4 Number of breeding pairs per location for the species Antarctic shag (AS; Phalacrocorax bransfieldensis), southern giant petrel (SGP; Macronectes giganteus), Cape petrel (CP; Daption capense), snowy sheathbill (SS; Chionis alba), South Polar skua (SPS; Stercorarius maccormicki), kelp gull (KG; Larus dominicanus) and Antarctic tern (AT; Sterna vittata), in the northern sector of the Danco Coast. Lambda (λ) represents the annual breeding population growth rate, N represent the breeding population size and s the season (s1: 1997/98 and s2: 2010/11).
|
AS |
SGP |
CP |
SS |
SPS |
KG |
AT |
| Locality |
Ns1 |
Ns2 |
λ
|
Ns1 |
Ns2 |
λ
|
Ns1 |
Ns2 |
λ
|
Ns1 |
Ns2 |
λ
|
Ns1 |
Ns2 |
λ
|
Ns1 |
Ns2 |
λ
|
Ns1 |
Ns2 |
λ
|
| Cape Herschel |
28 |
24 |
0.99 |
0 |
0 |
– |
2 |
0 |
0 |
1 |
1 |
1 |
2 |
0 |
0 |
2 |
4 |
1.05 |
0 |
10 |
– |
| Cierva Point |
0 |
0 |
– |
0 |
0 |
– |
7 |
3 |
0.94 |
2 |
1 |
0.95 |
145 |
166 |
1.01 |
158 |
73 |
0.94 |
45 |
57 |
1.02 |
| Kay Rock |
0 |
0 |
– |
0 |
0 |
– |
0 |
0 |
– |
0 |
0 |
– |
1 |
4 |
1.11 |
0 |
0 |
– |
0 |
0 |
– |
| Mar Rock |
9 |
0 |
0 |
0 |
0 |
– |
1 |
0 |
0 |
3 |
1 |
0.92 |
3 |
3 |
1 |
8 |
10 |
1.02 |
0 |
3 |
– |
| Moss Islands |
0 |
0 |
– |
35 |
42 |
1.01 |
28 |
17 |
0.96 |
3 |
4 |
1.02 |
10 |
26 |
1.08 |
120 |
70 |
0.96 |
15 |
19 |
1.02 |
| Midas Island |
21 |
21 |
1 |
0 |
7b
|
– |
0 |
0 |
– |
1 |
1 |
1 |
3 |
17 |
1.14 |
15 |
9 |
0.96 |
35 |
11b
|
0.91 |
| Sucia Point |
0 |
0 |
– |
47 |
68 |
1.03 |
0 |
0 |
– |
0 |
0 |
– |
25b
|
32 |
1.02 |
35 |
31 |
0.99 |
15 |
22 |
1.03 |
| Sterneck Island |
0 |
0 |
– |
5b
|
41 |
1.17 |
23 |
11 |
0.94 |
1b
|
2 |
1.05 |
2b
|
12 |
1.15 |
68 |
12 |
0.87 |
15 |
12 |
0.98 |
| Py Point |
22 |
34 |
1.03 |
0 |
0 |
– |
0 |
0 |
– |
1 |
4 |
1.11 |
0 |
5 |
– |
35 |
7 |
0.88 |
20 |
11 |
0.95 |
| Charles Point |
0 |
0 |
– |
0 |
0 |
– |
0 |
0 |
– |
0 |
1 |
– |
40b
|
31 |
0.98 |
105 |
98 |
0.99 |
15b
|
32 |
1.06 |
| Alcock Island |
2 |
0 |
0 |
5b
|
3b
|
0.96 |
7b
|
11 |
1.03 |
4 |
12 |
1.09 |
7b
|
19 |
1.08 |
37 |
70 |
1.05 |
20b
|
65 |
1.09 |
| Spring Point |
10a
|
0 |
0 |
0a
|
0 |
0 |
0a
|
0 |
– |
0a
|
0 |
– |
4a
|
12 |
1.13 |
25a,b
|
35 |
1.06 |
15a
|
0 |
0.93 |
| Sprightly Island |
|
0 |
|
|
0 |
– |
|
0 |
|
|
0 |
|
|
8 |
|
|
18 |
|
|
6 |
|
| Total |
92 |
79 |
0.99 |
92 |
161 |
1.04 |
68 |
42 |
0.96 |
16 |
27 |
1.04 |
242 |
335 |
1.02 |
608 |
437 |
0.97 |
195 |
248 |
1.02 |
| aSpring Point and Sprightly Island were grouped in Ns1.
|
| bPossible underestimation. |
Discussion
Spots censuses can give misleading impressions of the actual status of populations (Croxall et al. 1988). However, in order to understand ecological drivers for the population trends observed in seabirds, it is still necessary to have a regional vision of the status of the populations, and for that purpose, even opportunistic visits to colonies provide valuable information (Lynch et al. 2008). Species richness among the sites studied ranged between two and nine, with the Wilson′s storm petrel present in all localities. In general, the breeding populations of pygoscelids, southern giant petrels and snowy sheathbills increased, whereas those of Cape petrels and kelp gulls decreased. These increases and decreases could be attributed to the variability of the environment and/or different counting techniques used in other decades in association with different meteorological conditions. For this reason, arbitrarily and applying a more conservative approach, we only discuss here those species for which annual population growth rate (λ) was below 0.97 or above 1.03.
Population trends of pygoscelids penguins in the Antarctic Peninsula and associated islands have been studied by numerous authors (Woehler & Croxall 1997; Hinke et al. 2007; Sander, Balbao, Costa et al. 2007; Sander, Balbao, Polito et al. 2007; Carlini et al. 2009; Trivelpiece et al. 2011; Lynch, Ron et al. 2012; Lynch, William et al. 2012; among many others). The contrasting trends between species are of particular interest because as top predators, they integrate and/or magnify changes in the lower levels of the food chain. Thus, penguins, who have different affinities to sea ice, provided some of the first evidence linking changes in the physical environment to their biological responses (Fraser et al. 1992; Rombolá et al. 2003; Forcada et al. 2006; Trathan et al. 2007; Nicol et al. 2008; Forcada & Trathan 2009). Ice reduction will adversely impact the winter over survival of krill larvae and the reproductive output of female krill and as consequence diminish krill recruitment (Flores et al. 2012), which in turn impacts krill availability to top predators. Furthermore, sea-ice loss impacts directly on the wintering habitats of sea-ice dependent species like Adélie penguins (Pygoscelis adeliae), and favours an increase in ice-intolerant gentoo and chinstrap penguins (Mc Clintock et al. 2008). Climate warming also brings more years with more snow (Turner et al. 2005; Thomas et al. 2008). Snow accumulation, snow melting and solar radiation impact the microclimate of breeding colonies and influence the selection of nesting sites among Pygoscelis species (Trivelpiece & Fraser 1996; Boersma 2008; Lynch et al. 2009; Ainley 2010), so differences in the breeding chronology will determine which one is mainly affected. Despite all evidences described in the literature about how climate change is operating on Antarctic Peninsula and its consequences for penguin populations, our results on Pygoscelis penguins showed an increase. Though the Danco Coast area is not under pressure from krill fisheries (CCAMLR 2011), krill availability to top predators may vary by natural causes and/or may be influenced by sea-ice reduction and availability of winter habitats. The biological responses to sea-ice and climatic variability are often extremely complex and may differ regionally and locally (Stenseth et al. 2002; Forcada et al. 2006). Winter sea-ice extent in the western Antarctic Peninsula exhibits high interanual variability, but ice maxima are episodic and high-ice years are invariably followed by several low-ice years that together form a distinct series or cycle (Fraser & Hoffmann 2003). Future results including diet information and breeding phenology will be necessary to identify how climate change is operating at Danco Coast on penguin populations.
Overall, population trends of southern giant petrels varied between different localities on the Antarctic Peninsula and sub-Antarctic islands and in Patagonia, Argentina, linked to human activity (Chupin 1997; Nel et al. 2002; Pfeiffer & Peter 2004; Patterson et al. 2008), habitat loss or degradation (Parks and Wildlife Service and Biodiversity Conservation Branch 2007) or food availability (Patterson et al. 2008). In general, those populations located in areas with human disturbance (accumulation of garbage, pollution, tourism, fisheries, predation by introduced animals) are suffering a decline (Woehler et al. 2001; Patterson et al. 2008), while in areas with less disturbance their populations are increasing (Woehler et al. 2001; Lynch et al. 2008). Our study area is within or near ASPA No. 134, where visits (by scientists and tourists) are not frequent. The number of tourists who visited Cierva Cove during the study year was 1272 (IAATO 2011), constituting only 0.4% of total tourists visiting the Antarctic region. Increases in the southern giant petrel population size at Danco Coast could be related to the relatively undisturbed area.
Pairs of breeding sheathbills maintain foraging and breeding territories centred on colonies of breeding seabirds, usually penguins (Burger 1981). In these habitats, they feed on eggs, carrion, faeces and perform kleptoparisitism (Downes et al. 1959; Burger 1981; Weimerskirch 1989; Verheyden & Jouventin 1991). Therefore, it is highly probable that the increases in abundance of snowy sheathbills recorded in the present study are related to breeding population growth observed in pygoscelids. In contrast, the possible causes that could explain the decrease in the breeding populations of cape petrels and kelp gulls remain unknown.
The study area has a high species richness, both animal and vegetable (Dirección Nacional del Antártico 2002). In turn, the highest abundance of birds, primarily pygoscelids, was within ASPA No. 134. The singular topography, along with the abundance and diversity of species, provides favourable conditions for the formation of numerous microhabitats, providing an exceptional scientific value to the area (Dirección Nacional del Antártico 2002). The present study highlights the need to maintain basics monitoring studies.
Acknowledgements
The study was supported by the Dirección Nacional del Antártico, Instituto Antártico Argentino and the Dirección Antártica. We are grateful for the cooperation agreement between the Marine Mammal Laboratory of the Instituto Antártico Argentino and the University of New South Wales and Taronga Conservation Society Australia. We thank anonymous reviewers and Dr C. Patrick Doncaster for their constructive comments. Special thanks to J. Mennucci, H. Merlo Álvarez, F. Isla, V. Llampa, J. Gómez, F. Villalba, R.C. Larrea, M. Tucker, W.E. Varela, E.H. Toso, M.A. Schell, C. Asti and D. Slip.
References
Ainley
D.,
Russell
J.,
Jenouvrier
S.,
Woehler
E.,
Lyver
P.O.B.,
Fraser
W.R.
&
Kooyman
G.L. 2010.
Antarctic penguin response to habitat change as Earth's troposphere reaches 2°C above pre-industrial levels. Ecological Monographs 80,
49–66.
Publisher Full Text
Boersma
P.D. 2008.
Penguins as marine sentinels. BioScience 58,
597–607.
Publisher Full Text
Burger
A.E. 1981.
Time budget, energy needs and kleptoparasitism in breeding lesser sheathbills (Chionis minor). The Condor 83,
106–112.
Publisher Full Text
Carlini
A.R.,
Coria
N.R.,
Santos
M.M.,
Negrete
J.,
Juares
M.A.
&
Daneri
G.A. 2009.
Responses of Pygoscelis adeliae and P. papua populations to environmental changes at Isla 25 de Mayo (King George Island). Polar Biology 32,
1427–1433.
Publisher Full Text
Caswell
H. 1989. Matrix population models. Construction, analysis, and interpretation. Sunderland, MA: Sinauer Associates.
CCAMLR 2011. Krill fishery report: 2011 update. WG-EMM-11/5.
Hobart: Commission for the Conservation of Antarctic Marine Living Resources Secretariat.
Chambers
L.E.,
Devney
C.A.,
Congdon
B.C.,
Dunlop
N.,
Woehler
E.J.
&
Dann
P. 2011.
Observed and predicted effects of climate on Australian seabirds. Emu 111,
235–251.
Publisher Full Text
Chupin
I. 1997.
Human impact and breeding success in southern giant petrel (Macronectes giganteus) on King George Island (South Shetland Islands). Korean Journal of Polar Research 8,
113–116.
Cook
A.J.,
Fox
A.J.,
Vaughan
D.G.
&
Ferrigno
J.G. 2005.
Retreating glacier-fronts on the Antarctic Peninsula over the last 50 years. Science 22,
541–544.
Publisher Full Text
Croxall
J.P.,
McCann
T.S.,
Prince
P.A.
&
Rothery
P. 1988. Reproductive performance of seabirds and seals at South Georgia and Signy Island, South Orkney Islands, 1976–1987: implications for Southern Ocean monitoring studies. In
D.
Sahrhage (ed.): Antarctic Ocean and resource variability. Pp. 261–285.
New York: Springer.
Dirección Nacional del Antártico 2002. Plan de gestión de la Zona Antártica Especialmente Protegida No 134 Punta Cierva e islas offshore, Costa Danco, Península Antártica. (Management Plan for Antarctic Specially Protected Area No. 134 Cierva Point and offshore islands, Danco Coast, Antarctic Peninsula.)
Buenos Aires: Áreas Protegidas, Dirección Nacional del Antártico.
Downes
M.C.,
Ealey
E.H.M.,
Gwynn
A.M.
&
Young
P.S. 1959. The birds of Heard Island. ANARE Reports 51. Melbourne: Antarctic Division, Department of External Affairs.
Favero
M.,
Coria
N.R.
&
Beron
M.P. 2000.
The status of breeding birds at Cierva Point and surroundings, Danco Coast, Antarctic Peninsula. Polish Polar Research 21,
181–187.
Flores
H.,
van Franeker
J.A.,
Siegel
V.,
Haraldsson
M.,
Strass
V.,
Hubert Meesters
E.,
Bathmann
U.
&
Jan Wolff
W.
2012. The association of Antarctic krill Euphausia superba with the under-ice habitat. PLoS One
7(2), e31775, doi: 10.1371/journal.pone.0031775.
PubMed Abstract | PubMed Central Full Text | Publisher Full Text
Forcada
J.
&
Trathan
P.N. 2009.
Penguins responses to climate change in the Southern Ocean. Global Change Biology 7,
1618–1630.
Publisher Full Text
Forcada
J.,
Trathan
P.N.,
Reid
K.,
Murphy
E.J.
&
Croxall
J.P. 2006.
Contrasting population changes in sympatric penguin species in association with climate warming. Global Change Biology 12,
411–423.
Publisher Full Text
Fraser
W.R.
&
Hofmann
E. 2003.
A predator's perspective on causal links between climate change, physical forcing and ecosystem response. Marine Ecology Progress Series 265,
1–15.
Publisher Full Text
Fraser
W.R.,
Trivelpiece
W.Z.,
Ainley
D.G.
&
Trivelpiece
S.G. 1992.
Increases in Antarctic penguin populations: reduced competition with whales or a loss of sea ice due to environmental warming? Polar Biology 11,
525–531.
Publisher Full Text
Gille
S.T. 2002.
Warming of the Southern Ocean since the 1950s. Science 295,
1275–1277.
PubMed Abstract | Publisher Full Text
Hinke
J.T.,
Salwicka
K.,
Trivelpiece
S.G.,
Watters
G.M.
&
Trivelpiece
W.Z. 2007.
Divergent responses of Pygoscelis penguins reveal common environmental driver. Oecologia 153,
845–855.
PubMed Abstract | Publisher Full Text
IAATO (International Association of Antarctica tour Operators) 2011. Tourism statistics. Accessed on the internet at http://iaato.org/es/tourism-statistics on 22 January 2013.
Loeb
V.,
Siegel
V.,
Holm-Hansen
O.,
Hewitt
R.,
Fraser
W.,
Trivelpiece
W.
&
Trivelpiece
S. 1997.
Effects of sea-ice extent and krill or salp dominance on the Antarctic food web. Nature 387,
897–900.
Publisher Full Text
Lynch
H.J.,
Fagan
W.F.,
Naveen
R.,
Trivelpiece
S.G.
&
Tricelpiece
W.Z. 2009.
Timing of clutch initiation in Pygoscelis penguins on the Antarctic Peninsula: towards an improved understanding of off-peak census correction factors. CCAMLR Science 16,
149–165.
Lynch
H.J.,
Naveen
R.
&
Fagan
W.F. 2008.
Censuses of penguin, blue-eyed shag Phalacrocorax atriceps and southern giant petrel Macronectes giganteus populations on the Antarctic Peninsula, 2001–2007. Marine Ornithology 36,
83–97.
Lynch
H.J.,
Ron
N.,
Philip
N.T.
&
William
F.F. 2012.
Spatially integrated assessment reveals widespread changes in penguin populations on the Antarctic Peninsula. Ecology 93,
1367–1377.
PubMed Abstract | Publisher Full Text
Lynch
H.J.,
William
F.F.,
Ron
N.,
Trivelpiece
S.G.
&
Trivelpiece
W.Z. 2012.
Differential advancement of breeding phenology in response to climate may alter staggered breeding among sympatric pygoscelid penguins. Marine Ecology Progress Series 454,
135–145.
Publisher Full Text
Mc Clintock
J.,
Ducklow
H.
&
Fraser
W. 2008.
Ecological responses to climate change on the Antarctic Peninsula. The Scientific Research Society 96,
302–310.
Muller-Schwarze
C.
&
Muller-Schwarze
D. 1975.
A survey of twenty-four rookeries of pygoscelid penguins in the Antarctic Peninsula region.
In B. Stonehouse (ed.): The biology of penguins. Pp. 309–320. London: Macmillan.
Nel
D.C.,
Ryan
P.G.,
Crawford
R.J.M.,
Cooper
J.
&
Huyser
O.A.W. 2002.
Population trends of albatrosses and petrels at sub-Antarctic Marion Island. Polar Biology 25,
81–89.
Nicol
S.,
Clarke
J.,
Romaine
S.J.,
Kawaguchi
S.,
Williams
G.
&
Hosie
G.W. 2008.
Krill (Euphausia superba) abundance and Adélie penguin (Pygoscelis adeliae) breeding performance in the waters off the Béchervaise Island colony, East Antarctica in 2 years with contrasting ecological conditions. Deep-Sea Research Part II 55,
540–557.
Publisher Full Text
Novatti
R. 1978. Notas ecológicas y etológicas sobre las aves de Cabo Primavera, Costa de Danco, Península Antártica. (Ecological and ethological notes on birds in Spring Point, Danco Coast, Antarctic Peninsula.) Contribución Instituto Antártico Argentino 237.
Buenos Aires: Argentine Antarctic Institute.
Parks and Wildlife Service and Biodiversity Conservation Branch 2007. Plan for the eradication of rabbits and rodents on Subantarctic Macquarie Island.
Hobart: Parks and Wildlife Service, Dept. of Tourism, Arts and the Environment, and Biodiversity Conservation Branch, Dept. of Primary Industries and Water.
Patterson
D.L.,
Woehler
E.J.,
Croxall
J.P.,
Cooper
J.,
Poncet
S.,
Peter
H.-U.,
Hunter
S.
&
Fraser
W.R. 2008.
Breeding distribution and population status of the northern giant petrel Macronectes halli and the southern giant petrel M. Giganteus. Marine Ornithology 36,
115–124.
Pfeiffer
S.
&
Peter
H.U. 2004.
Ecological studies toward the management of an Antarctic tourist landing site (Penguin Island, South Shetland Islands). Polar Record 40,
345–353.
Publisher Full Text
Poncet
S.
&
Poncet
J. 1987.
Censuses of penguin populations of the Antarctic Peninsula, 1983–87. British Antarctic Survey Bulletin 77,
109–129.
Quintana
R.D.,
Cirelli
V.
&
Orgeira
J.L. 1998.
Abundance and spatial distribution of bird populations at Cierva Point, Antarctic Peninsula. Marine Ornithology 28,
21–27.
Rombolá
E.,
Marschoff
E.
&
Coria
N. 2003.
Comparative study of the effects of the late pack-ice break-off on chinstrap and Adélie penguins’ diet and reproductive success at Laurie Island, South Orkney Islands, Antarctica. Polar Biology 26,
41–48.
Sander
M.,
Balbao
T.C.,
Costa
E.S.,
Dos Santos
C.R.
&
Petra
M.V. 2007.
Decline of the breeding population of Pygoscelis antarctica and Pygoscelis adeliae on Penguin Island, South Shetlands, Antarctica. Polar Biology 30,
651–664.
Publisher Full Text
Sander
M.,
Balbao
T.C.,
Polito
M.J.,
Costa
E.S.
&
Carneiro
A.P.B. 2007.
Recent decrease in chinstrap penguin (Pygoscelis antarctica) populations at two of Admiralty Bay's islets on King George Island, South Shetland Islands, Antarctica. Polar Biology 30,
659–661.
Publisher Full Text
Siegel
V.
&
Loeb
V. 1995.
Recruitment of Antarctic krill Euphausia superba and possible causes for its variability. Marine Ecology Progress Series 123,
45–56.
Publisher Full Text
Smith
R.C.,
Ainley
D.,
Baker
K.,
Domack
E.,
Emslie
S.,
Fraser
S.,
Kennet
A.,
Mosley-Thomson
E.,
Stammerjohn
S.
&
Vernet
M. 1999.
Marine ecosystem sensitivity to climate change. BioScience 49,
393–404.
Publisher Full Text
Stenseth
N.C.,
Mysterud
A.,
Ottersen
G.,
Hurrell
J.W.,
Chan
K-S.
&
Lima
M. 2002.
Ecological effects of climate fluctuations. Science 297,
1292–1296.
PubMed Abstract | Publisher Full Text
Thomas
E.R.,
Marshall
G.J.
&
McConnell
J.R.
2008. A doubling in snow accumulation in the western Antarctic Peninsula since 1850. Geophysical Research Letters
35, L01706, doi: 10.1029/2007GL032529.
Publisher Full Text
Trathan
P.N.,
Forcada
J.
&
Murphy
E.J. 2007.
Environmental forcing and Southern Ocean marine predator populations: effects of climate change and variability. Philosophical Transactions of the Royal Society B 362,
2351–2365.
Publisher Full Text
Trivelpiece
W.Z.
&
Fraser
W.R. 1996.
The breeding biology and distribution of Adelie penguins: adaptations to environmental variability.
In R. Ross (ed.): Foundations for ecological research west of the Antarctic Peninsula. Pp. 273–285. Washington, DC: American Geophysical Union.
Trivelpiece
W.Z.,
Hinke
J.T.,
Miller
A.K.,
Reiss
C.S.,
Trivelpiece
S.G.
&
Watters
G.M. 2011.
Variability in krill biomass links harvesting and climate warming to penguin population changes in Antarctica. Proceedings of the National Academy of Sciences of the United States of America 108,
7625–7628.
PubMed Abstract | PubMed Central Full Text | Publisher Full Text
Turner
J.,
Lachlan-Cope
T.,
Colwell
S.
&
Marshall
G. 2005.
A positive trend in western Antarctic Peninsula precipitation over the last 50 years reflecting regional and Antarctic-wide atmospheric circulation changes. Annals of Glaciology 41,
85–91.
Publisher Full Text
Vaughan
D.G.,
Marshall
G.J.,
Connolley
W.M.,
King
J.C.
&
Mulvaney
R. 2001.
Climate change: devil in the detail. Science 293,
1777–1779.
PubMed Abstract | Publisher Full Text
Vaughan
D.G.,
Marshall
G.J.,
Connolley
W.M.,
Parkinson
C.,
Mulvaney
R.,
Hodgson
D.A.,
King
J.C.,
Pudsey
C.J.
&
Turner
J. 2003.
Recent rapid regional climate warming on the Antarctic Peninsula. Climate Change 60,
243–274.
Publisher Full Text
Verheyden
C.
&
Jouventin
P. 1991.
Over-wintering strategies of the lesser sheathbill Chionis minor in an impoverished and insular environment. Oecología 86,
132–139.
Weimerskirch 1989.
The avifauna of the Kerguelen Islands. Emu 89,
15–29.
Publisher Full Text
Woehler
E.J. 1993. The distribution and abundance of Antarctic and Subantarctic penguins. Cambridge: Scientific Committee on Antarctic Research.
Woehler
E.J.,
Cooper
J.,
Croxall
J.P.,
Fraser
W.R.,
Kooyman
G.L.,
Millar
G.D.,
Nel
D.C.,
Patterson
D.L.,
Peter
H.-U.,
Ribic
C.A.,
Salwicka
K.,
Trivelpiece
W.Z.
&
Weimerskirch
H. 2001. A statistical assessment of the status and trends of Antarctic and Subantarctic seabirds. Cambridge: Scientific Committee on Antarctic Research.
Woehler
E.J.
&
Croxall
J.P. 1997.
The status and trends of Antarctic and sub-Antarctic seabirds. Marine Ornithology 25,
43–66.