RESEARCH/REVIEW ARTICLE
Contrasting strategies of resistance vs. tolerance to desiccation in two polar dipterans
M.J. Everatt,1 P. Convey,2,3 M.R. Worland,2 J.S. Bale1 & S.A.L. Hayward1
1School of Biosciences, University of Birmingham, Edgbaston, Birmingham B15 2TT, UK
2British Antarctic Survey, Natural Environment Research Council, High Cross, Madingley Road, Cambridge CB3 0ET, UK
3National Antarctic Research Center, IPS Building, University Malaya, 50603 Kuala Lumpur, Malaysia
Abstract
Low water availability is one of the principal stressors for terrestrial invertebrates in the polar regions, determining the survival of individuals, the success of species and the composition of communities. The Arctic and Antarctic dipterans Heleomyza borealis and Eretmoptera murphyi spend the majority of their biennial life cycles as larvae, and so are exposed to the full range of environmental conditions, including low water availability, over the annual cycle. In the current study, the desiccation resistance and desiccation tolerance of larvae were investigated, as well as their capacity for cross-tolerance to temperature stress. Larvae of H. borealis showed high levels of desiccation resistance, only losing 6.9% of their body water after 12 days at 98.2% relative humidity (RH). In contrast, larvae of E. murphyi lost 46.7% of their body water after 12 days at the same RH. Survival of E. murphyi larvae remained high in spite of this loss (>80% survival). Following exposure to 98.2% RH, larvae of E. murphyi showed enhanced survival at −18°C for 2 h. The supercooling point of larvae of both species was also lowered following prior treatment at 98.2% RH. Cross-tolerance to high temperatures (37 or 38.5°C) was not noted following desiccation in E. murphyi, and survival even fell at 37°C following a 12-day pre-treatment. The current study demonstrates two different strategies of responding to low water availability in the polar regions and indicates the potential for cross-tolerance, a capacity which is likely to be beneficial in the ever-changing polar climate.
Keywords
Acclimation; dipteran; supercooling point; temperature; cross-tolerance.
Correspondence
Matthew John Everatt, School of Biosciences, University of Birmingham, Edgbaston, Birmingham B15 2TT, UK. E-mail: mjemarue@hotmail.co.uk
(Published: 22 May 2014)
Polar Research 2014. © 2014 M.J. Everatt et al. This is an Open Access article distributed under the terms of the Creative Commons Attribution-Noncommercial 3.0 Unported License (http://creativecommons.org/licenses/by-nc/3.0/), permitting all non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Citation: Polar Research 2014, 33, 22963, http://dx.doi.org/10.3402/polar.v33.22963
Insects, which are largely of small size, have a high surface area to volume ratio and are vulnerable to water loss (Gibbs et al. 1997). Injuries resulting from the loss of water include protein denaturation and unwanted macromolecular interactions, crystalline to gel membrane phase transitions, oxidative damage and mechanical stress (Danks 2000). In order to protect against these injuries, invertebrates generally adopt one of two strategies, desiccation resistance or desiccation tolerance. The capacity to prevent water loss from the body (desiccation resistance) varies greatly among invertebrates and has led to three species classifications, namely hygric species, which have little or no control over their water loss, and transitional and mesic species, which are increasingly able to regulate the loss of body water (Danks 2000). The mesic status of invertebrates like the Antarctic mite Alaskozetes antarcticus is largely achieved through lowered cuticular permeability (e.g., Benoit, Yoder et al. 2007), though the regulation of water is also achieved in other invertebrates using methods of freezing (Convey 1992), membrane alteration and metabolic suppression (Michaud et al. 2008) and/or specialized respiration (Danks 2000). In hygric species, the loss of water is tolerated. Dendrobaena octaedra (earthworm) cocoons (Holmstrup & Zachariassen 1996) and larvae of the Antarctic dipteran Belgica antarctica (Hayward et al. 2007) are able to endure >75% loss of their water content, while some insects, such as the dipteran Polypedilum vanderplaanki and many nematodes and tardigrades, are able to survive the loss of virtually all their osmotically active water, employing the tactic of anhydrobiosis (Crowe & Madin 1975; Wharton & Barclay 1993; Watanabe et al. 2002; Wharton 2003, 2011; Hengherr et al. 2010). Molecular mechanisms underpinning desiccation tolerance include the accumulation of polyhydric alcohols and sugars (Benoit, Lopez-Martinez et al. 2007; Hengherr et al. 2008), the utilization of HSP and LEA proteins (Bahrndorff et al. 2009; Lopez-Martinez et al. 2009; Popovic et al. 2011), shifts in metabolism (Danks 2000; Li et al. 2009), membrane remodelling (Lopez-Martinez et al. 2009), oxidative damage repair (Lopez-Martinez et al. 2008) and cytoskeletal reorganization (Li et al. 2009; Lopez-Martinez et al. 2009).
Low water availability is seen as being one of two principal stressors to terrestrial invertebrates in the polar regions, with the other being low temperature (Cannon & Block 1988; Convey 1996; Strathdee & Bale 1998; Block et al. 2009). In winter, water is locked up as ice and is inaccessible to invertebrates (Block et al. 2009), while in summer, evaporation of meltwater can lead to drought (Kennedy 1993). In some areas, such as the McMurdo Dry Valleys, soil water content can be as little as 2% (Treonis & Wall 2005). The Antarctic dipteran Eretmoptera murphyi and the High-Arctic dipteran Heleomyza borealis also experience aridity in their respective habitats. The larval stages of these two species comprise the majority of the life cycle duration, and thus experience the full spectra of environmental conditions over the annual cycle (Convey & Block 1996; Worland et al. 2000). Eretmoptera murphyi is locally highly abundant in the sub-Antarctic island of South Georgia and, since its introduction onto Signy Island (maritime Antarctic) in the 1960s, it has spread to occupy an area >2000 m2, with densities as high as 410 000 ind. m−2 (Hughes & Worland 2010). Worland (2010) has shown that E. murphyi larvae possess good desiccation tolerance, but low desiccation resistance. Heleomyza borealis is also abundant at certain High-Arctic sites when found in association with bird colonies, but its desiccation tolerance has not been assessed thoroughly.
Because injuries to invertebrates, such as protein impairment and loss of membrane fluidity, result from desiccation as well as from other stresses like low temperature, the physiological adaptations induced in response to these stresses are analogous, or at least complementary (Ring & Danks 1998; Bayley et al. 2001). For example, the desaturation of membranes, up-regulation of heat shock proteins and the accumulation of polyols and sugars occur after both desiccation and low temperature treatments (Bayley et al. 2001; Holmstrup et al. 2002; Bahrndorff et al. 2007; Benoit et al. 2009). It therefore follows that a sub-lethal desiccation exposure can potentially afford protection for an invertebrate subsequently exposed to low temperatures. This phenomenon is termed cross-tolerance and has been observed in a number of organisms, though primarily in Diptera and Collembola (e.g., Holmstrup et al. 2002; Elnitsky et al. 2008; Levis et al. 2012). In the collembolan Megaphorura arctica desiccation in the presence of ice lowers the supercooling point (SCP) to such an extent that the collembolan is able to survive the low temperatures of the Arctic winter (Worland et al. 1998). This strategy, termed cryoprotective dehydration, is now seen to be fairly common, having been described in an increasing number of invertebrates (e.g., Pedersen & Holmstrup 2003; Elnitsky et al. 2008; Smith et al. 2008; Sørensen & Holmstrup 2011). Cross-tolerance also works independently of the SCP. In the freeze-tolerant dipteran B. antarctica survival was improved by 90% at −10°C following 48 h at 98% relative humidity (RH) and by 60% at −15°C following the loss of 50% of its body water (Benoit et al. 2009). Invertebrates that experience complete desiccation or anhydrobiosis are also conferred improved low temperature tolerance, and the extent to which it is improved is usually greater than in partially desiccated animals like B. antarctica (e.g., Ramløv & Westh 1992; Sømme & Meier 1995; Shuker 2001).
Climate change is leading to warmer summers in the polar regions, with evidence of increasing exposure to drought (Convey et al. 2003; Convey et al. 2009; Turner et al. 2009). Exploration of cross-tolerance between desiccation and low and high temperature in two additional dipteran species, therefore, provides further insight into how polar terrestrial invertebrates tolerate extreme conditions currently and may indicate how they will cope with climate warming in future.
Materials and methods
Insect collection and storage conditions
Summer acclimatized individuals of Heleomyza borealis were collected from the moss-covered slopes at Krykkjefjellet and Blomstrandhalvøya, near Ny-Ålesund, Spitsbergen, Svalbard (78°55′N, 11°56′E) in August 2011. Summer acclimatized individuals of Eretmoptera murphyi were collected from soil and moss on Signy Island (60°S, 45°W) close to the British Antarctic Survey Signy Research Station between January and March 2012. They were transported to the University of Birmingham under refrigerated conditions and then held in plastic boxes containing substratum from the site of collection at 4°C (0:24 L:D). The duration of travel was approximately two days from the Arctic and two months from the Antarctic. Numbers of H. borealis were limited and hence it was not possible to assess for cross-tolerance to high and low temperatures, except with respect to their SCPs.
Water balance and desiccation tolerance
Bayley & Holmstrup (1999) highlighted the importance of performing desiccation experiments at more ecologically relevant RH values, and in particular those close to the wilting point of plants (ca. 98.9% RH). The specific RH of 98.2% used here was produced using 150 ml of NaCl solution (31.60 g NaCl L−1) in a plastic container. The RH was verified as being stable using a Hygrochron temperature and humidity logger iButton (Maxim, San Jose, CA, USA). Controls were maintained at 100% RH using purified water and were given access to water. Dipteran larvae were placed in small glass containers, covered with nylon gauze, which were then placed inside the plastic containers, and sealed with a tight fitting lid. Following Raoult’s law, the air inside the closed system quickly equilibrated with the aqueous solution used.
Three replicates of 10 individuals of each species were removed from 98.2% RH at set intervals over a 12-day period (6 h, 2, 4, 8 and 12 days). Dipteran larvae were subsequently transferred into plastic universal tubes containing moist plaster of Paris and given substrate and water. Survival, defined as larvae which either moved spontaneously or in response to gentle contact stimulus, was assessed 72 h after treatment. The larvae were also weighed prior to desiccation, upon removal from each desiccation treatment, and following drying to constant mass at 60°C over 24 h. From these values, initial water content and percentage water loss or gain were calculated (see Hayward et al. 2007).
Desiccation induced low temperature tolerance
Effect of desiccation on the SCP
Individuals of H. borealis and E. murphyi were held at 98.2% RH for either 6 h, 2, 4, 8 or 12 days (only 12 days for H. borealis) prior to experimental treatment. Fifteen larvae were placed in contact with a thermocouple, within Beem capsules, in glass test tubes plugged with sponge, inside an alcohol bath (Haake Phoenix II C50P, Fisher Scientific UK Ltd, Loughborough, UK). Larvae were subsequently cooled from 4 to −30°C at 0.5 min−1. SCPs, defined as the temperature at the onset of the freezing exotherm, were identified using an eight channel datalogger interfaced to a computer and recorded using Picolog Recorder software (Pico Technology Limited, St. Neots, UK; see Hawes et al. 2006).
Lower discriminating temperature
The temperature at which 10–20% survival occurred (Lee et al. 1987) was determined by cooling three replicates of 10 larvae at 0.2°C min−1 to progressively lower sub-zero temperatures (−15 to −19°C) for 2 h, before being re-warmed to the rearing temperature (4°C) at the same rate. Larvae were placed in Eppendorf tubes, inside glass test tubes plugged with sponge, in an alcohol bath prior to each experimental treatment. Control groups were handled, and exposed, in the same way at 4°C. The temperature experienced by the larvae was measured by placing a thermocouple within an identical Eppendorf tube into one of the glass test tubes. At the end of each experimental treatment, the larvae were rapidly transferred (over ice) from the Eppendorf tubes into plastic universal tubes containing moist plaster of Paris and substratum, and returned to the rearing conditions. Survival was assessed as described previously. The highest temperature at which survival was between 10 and 20% after 72 h recovery was defined as the discriminating temperature.
Effect of desiccation on low temperature tolerance
Larvae of E. murphyi only were held at 98.2% RH for 6 h, 2, 4, 8 or 12 days prior to experimental treatment. Three replicates of 10 larvae were subsequently cooled at 0.2°C min−1 to the discriminating temperature and held for 2 h before being re-warmed to the rearing temperature at the same rate. Larvae collection and handling, controls, thermocouple use, recovery and survival assessment were as described previously.
Desiccation-induced heat tolerance
Higher discriminating temperature
The temperature at which 10–20% survival occurred was determined by warming three replicates of 10 individuals at 0.2°C min−1 to progressively higher temperatures (30–40°C) for 2 h, before being re-cooled to the rearing temperature at the same rate. Larvae collection and handling, controls, thermocouple use, recovery and survival assessment were as described previously. The lowest temperature at which survival was between 10 and 20% after 72 h recovery was defined as the discriminating temperature. The lowest temperature at which survival was between 80 and 90% was also used to assess whether survival was lowered by a prior desiccation exposure.
Effect of desiccation on high temperature tolerance
Larvae of E. murphyi were held at 98.2% RH for 6 h, 2, 4, 8 and 12 days prior to experimental treatment. Three replicates of 10 larvae were subsequently warmed at 0.2°C min−1 to the discriminating temperature and the 80–90% survival temperature. Larvae were held for 2 h and cooled to the rearing temperature at the same rate. Larvae collection and handling, controls, thermocouple use, recovery and survival assessment were as described previously.
Statistical analysis
The Kolmogorov-Smirnov test was used to check for normality in the survival, SCP and percentage water loss data. Normally distributed data were analysed using analysis of variance (ANOVA) and Tukey’s multiple range test; data that were not normally distributed were analysed using the Kruskal-Wallis test.
Results
Water balance and desiccation tolerance
Larvae of Heleomyza borealis were significantly more desiccation resistant than those of Eretmoptera murphyi (Fig. 1). After 12 days, larvae of H. borealis had lost only 6.9% of their water content, as compared with 46.7% in larvae of E. murphyi. Water loss rate was not constant in larvae of E. murphyi; between 2 and 4 days, they regained 13.6% of their initial water content, before losing water rapidly again thereafter. Survival following 6 h, 2 days, 4 days, 8 days and 12 days at 98.2% RH was high in larvae of both H. borealis and E. murphyi, and was not significantly different from survival in the control (Fig. 2).
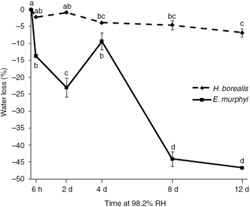
Fig. 1
Percentage water loss or gain of larvae of H. borealis and E. murphyi following exposure to 98.2% relative humidity (RH) for 6 h, 2 days, 4 days, 8 days and 12 days. Means±standard error of the mean are presented for three replicates of 10 individuals. Means with the same letter are not significantly different within each species group at p<0.05 (Tukey’s multiple range test).
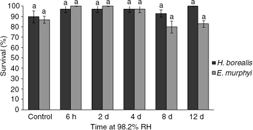
Fig. 2
Survival (%) of larvae of H. borealis and E. murphyi following exposure to 98.2% relative humidity (RH) for 6 h, 2 days, 4 days, 8 days and 12 days. Means±standard error of the mean are presented for three replicates of 10 individuals. Survival was assessed 72 h after treatment. Means with the same letter are not significantly different from the control within each species group at p<0.05 (Tukey’s multiple range test).
Desiccation-induced cold tolerance
Effect of desiccation on the SCP
Prior exposure to 98.2% RH significantly lowered the SCP in larvae of both species (Table 1). In larvae of H. borealis the SCP fell by 1.6°C after 12 days at 98.2% RH, while in larvae of E. murphyi the SCP fell by up to 2.5°C.
Table 1 H. borealis and E. murphyi larval supercooling points (SCPs) following exposure to 98.2% relative humidity (RH) for 6 h, 2 days, 4 days, 8 days and 12 days (only 12 days for H. borealis). Means±standard error of the mean are presented for 15 replicates. Asterisks indicate a treatment significantly different from the control (0 h) at p<0.05 (Tukey’s multiple range test).
| |
SCP (°C) |
| Species |
0 h |
6 h |
2 days |
4 days |
8 days |
12 days |
| H. borealis
|
−7.70±0.28 |
– |
– |
– |
– |
−9.29±0.38* |
| E. murphyi
|
−5.05±0.29 |
−6.16±0.11 |
−6.21±0.29 |
−5.36±0.40 |
−7.52±0.22* |
−6.74±0.46* |
Lower discriminating temperature and the effect of desiccation on low temperature tolerance
Survival of E. murphyi larvae declined gradually following exposure to progressively lower temperatures (Fig. 3a). The discriminating temperature (20% survival) was determined to be −18°C. At this temperature, survival of E. murphyi larvae was raised following all acclimation treatments (6 h, 2 days, 4 days, 8 days and 12 days) at 98.2% RH (Fig. 3b). The increase in survival was significant following 4 days.
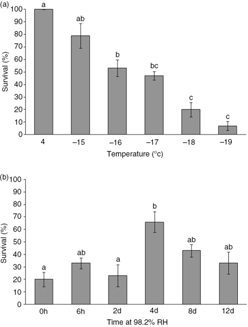
Fig. 3
Survival (%) of larvae of E. murphyi (a) after exposure to progressively lower sub-zero temperatures (−15 to −19°C) for 2 h, and (b) after exposure to −18°C, following prior exposure to 98.2% relative humidity (RH) for 6 h, 2 days, 4 days, 8 days and 12 days. Means±standard error of the mean are presented for three replicates of 10 individuals. Survival was assessed 72 h after treatment. Means with the same letter are not significantly different at p<0.05 (Tukey’s multiple range test).
Desiccation-induced heat tolerance
Higher discriminating temperature and the effect of desiccation on high temperature tolerance
Survival of E. murphyi larvae remained at 100% up to 35°C, but declined rapidly at temperatures near to the upper lethal temperature (ULT), falling by 80% between 37 and 40°C (Fig. 4a). The discriminating temperatures were determined to be 38.5 and 37°C, giving 20 and 80% survival, respectively. At 37°C, survival of E. murphyi larvae was unchanged at around 80% following 2, 4 and 8 days at 98.2% RH, but declined to 55% after 12 days (Fig. 4b). There was also no significant difference between non-acclimated and acclimated larvae of E. murphyi at 38.5°C.
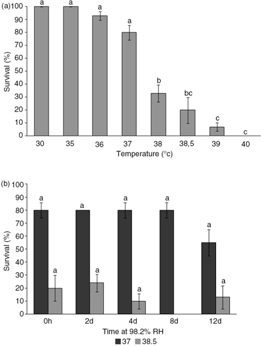
Fig. 4
Survival (%) of larvae of E. murphyi (a) after exposure to progressively higher temperatures (30–40°C) for 2 h and (b) after exposure to 37 or 38.5°C, following prior exposure to 98.2% relative humidity (RH) for 6 h, 2 days, 4 days, 8 days and 12 days. Means±standard error of the mean are presented for three replicates of 10 individuals. Survival was assessed 72 h after treatment. Means with the same letter are not significantly different across temperature treatments (a) and between temperature treatments (b) at p<0.05 (Tukey’s multiple range test).
Discussion
Desiccation resistance
Water availability is limited for much of the year in the Arctic and Antarctic (Strathdee & Bale 1998; Block et al. 2009), and polar terrestrial invertebrates respond with one of two strategies, desiccation resistance or desiccation tolerance. Our data suggest that the Arctic dipteran Heleomyza borealis responds through desiccation resistance, while the Antarctic species Eretmoptera murphyi tolerates substantial desiccation (Figs. 1, 2). The rate of water loss in E. murphyi was seven times more rapid than in H. borealis and was similar to that reported by Worland (2010) at 88% RH. The closely related dipteran Belgica antarctica which also inhabits the maritime Antarctic, likewise shows a high rate of water loss. When exposed to 98% RH, larvae of this dipteran took around 5 days to lose 50%, and 8–10 days to lose over 60%, of their body water (Benoit, Lopez-Martinez et al. 2007).
There is therefore a clear difference in the level of desiccation resistance between H. borealis and the Antarctic Diptera E. murphyi and B. antarctica. The physiology of the cuticular layer between these species provides a possible, albeit unexplored, explanation for the difference, and lowered cuticular permeability is a widespread adaptation that invertebrates use to raise their desiccation resistance (Danks 2000). Invertebrates achieve this by increasing the number and/or length of hydrocarbons or hydrophobic molecules in the wax layer surrounding their cuticle, resulting in tighter packing and a greater reduction of water loss rate (Gibbs et al. 1997; Benoit, Yoder et al. 2007; Speight et al. 2008). Benoit, Lopez-Martinez et al. (2007) showed that the length of hydrocarbons increased in desiccated larvae of B. antarctica, but this change was only slight and there was no change in the number of hydrocarbons. Eretmoptera murphyi is closely related to B. antarctica (Allegrucci et al. 2006; Allegrucci et al. 2012) and may therefore possess similar physiological adaptations. We speculate that the initial composition and change in the cuticle layer is more biased towards a greater number and length of hydrocarbons in H. borealis than either Antarctic dipteran. Differences in melanization between the two Diptera may also offer an explanation for the differing levels of resistance in the current study, as has been shown between Drosophila (Parkash, Rajpurohit et al. 2008; Parkash, Ramniwas et al. 2008; Parkash et al. 2009; Parkash et al. 2012; Ramniwas et al. 2013).
It should also be noted that 98.2% RH may be a sufficiently high humidity that larvae of H. borealis are able to absorb water from the atmosphere. This may also underlie our observation that, between 2 and 4 days’ exposure at 98.2% RH, larvae of E. murphyi exhibited reduced water loss and even rehydration. Rehydration has also been observed in other species. In the collembolan Folsomia candida nearly all of the water lost initially at 98.2% RH was recovered within 5–7 days, despite being continually held at 98.2% RH (Bayley & Holmstrup 1999). As confirmed by microarray, this recovery was supplemented by accumulating and synthesising myo-inositol, glucose and trehalose, which allowed the collembolan to become hyperosmotic to the environment and absorb moisture (Timmermans et al. 2009). An analogous response may be present in E. murphyi. However, such a response has not been observed in the closely related B. antarctica, which was unable to absorb water from the atmosphere at any RH, except complete saturation, i.e., 100% RH (Benoit, Lopez-Martinez et al. 2007; Hayward et al. 2007).
Desiccation tolerance
Heleomyza borealis showed >90% survival following 12 days of desiccation (Fig. 2). However, because water loss was so slight, even after 12 days at 98.2% RH, larvae cannot be said to have tolerated desiccation. Instead, it suggests that larvae of H. borealis are able to survive well under ecologically relevant relative humidities using a desiccation resistance strategy. Conversely, larvae of E. murphyi tolerated desiccation, having shown considerable water loss, but also survival, following 12 days at 98.2% RH (Fig. 2). Belgica antarctica also principally uses a desiccation tolerance strategy and has been shown to survive well following a 75% loss of initial water content (Benoit, Lopez-Martinez et al. 2007; Hayward et al. 2007).
One means of tolerating desiccation is through possessing high initial water content, as an organism must subsequently lose more water before reaching a point at which damage occurs or energy intensive mechanisms are induced (Hayward et al. 2007). This argument is reinforced by the increased water content observed in selected desiccation tolerant lines of Drosophila melanogaster (Gibbs 1997). In E. murphyi larvae, the initial water content was high, averaging 74.3% (73.28–75.40%) of body mass (Benoit et al. 2009). We did not assess osmotically active water in this study, though B. antarctica is known to have very high osmotically active water content relative to temperate species (Hayward et al. 2007). Once considerable water loss does occur, as was the case in the current study, the potential for injury is great and an organism must adapt accordingly. Injuries that result from desiccation include protein denaturation and unwanted macromolecular interactions (Benoit et al. 2009), crystalline to gel phase transitions (Hazel 1995), oxidative damage (Lopez-Martinez et al. 2008) and mechanical stress (Li et al. 2009). The responses of B. antarctica in this regard have been particularly well studied. Larvae accumulate glycerol and trehalose, which are suggested as being replacements for lost water and/or an aid to the production of amorphous sugar glasses (Danks 2000; Benoit, Lopez-Martinez et al. 2007; Michaud et al. 2008; Bahrndorff et al. 2009; Benoit et al. 2009; Hengherr et al. 2009; Clarke et al. 2013). Protein denaturation is also ameliorated via the up-regulation of HSPs in response to desiccation (Lopez-Martinez et al. 2009; Teets et al. 2012), and the fluidity of the membrane maintained using enzymes such as Δ9 FAD desaturase (Lopez-Martinez et al. 2009). Further physiological mechanisms induced in response to desiccation include oxidative damage repair through the accumulation of antioxidants (Lopez-Martinez et al. 2008), the minimization of mechanical stress via the restructuring of the cytoskeleton (Li et al. 2009), the inhibition of apoptosis through the regulation of autophagy and the suppression of metabolism (Teets et al. 2012). Larvae of B. antarctica therefore possess a suite of physiological responses against injuries resulting from desiccation. It is possible that E. murphyi possesses similar physiological adaptations that underlie its high level of desiccation tolerance. Indeed, the capacity to which they respond to temperature is very similar (Lee et al. 2006; Everatt et al. 2012).
Desiccation-induced cross-tolerance
Low temperatures
Survival of E. murphyi at −18°C was significantly raised following desiccation at 98.2% RH (Fig. 3b). Greater survivorship at low temperatures, following pre-exposure to unsaturated conditions, has also been observed for a number of other invertebrates, including B. antarctica (Benoit et al. 2009) and the springtails Cryptopygus antarcticus (Elnitsky et al. 2008; Everatt, Worland et al. 2013) and Folsomia candida (Holmstrup et al. 2002). Cross-tolerance is thought to occur between desiccation and low temperature because injuries that result from the two stresses are similar. Consequently, the physiological mechanisms induced in response to desiccation and low temperatures are often analogous (e.g., Bayley et al. 2001), and act in concert to give greater protection. Even a mild desiccation treatment resulting in 6–10% water loss has been shown to confer significant gains in cold tolerance in the goldenrod gall fly, Eurosta solidaginis (Levis et al. 2012). Interestingly, survival of −18°C was highest after 4 days at 98.2% RH and not after longer durations of 8 and 12 days (Fig. 3b). This corresponds with the time period at which larvae of E. murphyi exhibited rehydration (Fig. 1), suggesting physiological processes associated with rehydration provide cold tolerance, and that these are additive to the protection provided by those solely concerned with desiccation tolerance.
An effect on the SCP was also observed in the current study for both H. borealis and E. murphyi. Following a pre-exposure to 98.2% RH, the SCP was significantly reduced (Table 1). Both dipteran species are freeze-tolerant and it is therefore preferable for extracellular ice formation to take place at higher sub-zero temperatures, as it occurs more slowly and decreases the chance of tissue damage (Worland & Block 1999). The lowering of the SCP in H. borealis and E. murphyi was therefore more likely a by-product of, rather than an adaptation to, desiccation. Water loss passively increases the concentration of solutes already present and results in the colligative lowering of the SCP (e.g., Holmstrup & Zachariassen 1996). The dipteran larvae were also starved during the desiccation treatments and, during periods of starvation, ice-nucleating gut contents may be removed, reducing the likelihood of ice formation (Sømme & Block 1982; Cannon & Block 1988). Worland et al. (2006) also reported that time spent without access to food on water surfaces lowered the SCP of the collembolan Ceratophysella denticulata.
High temperatures
Prior exposure to desiccation at 98.2% RH had either no effect or a negative effect on the heat tolerance of E. murphyi larvae (Fig. 4b). Unlike low temperatures, injuries incurred as a result of high temperatures are dissimilar to those of desiccation, and physiological defences mounted in response to desiccation are therefore also different, and could even be conflicting. Consequently, little protection is afforded by prior acclimation to desiccation. A similar response has been observed in nematodes (Holmstrup & Zachariassen 1996) and Collembola (Everatt, Worland et al. 2013). However, improved heat tolerance has been noted in other invertebrates, particularly those which are anhydrobiotic (e.g., Hinton 1951, 1960; Sakurai et al. 2008). It is speculated that anhydrobiotic organisms, because of their tendency to vitrify, are less susceptible to injuries in general and that conflicting injuries are therefore less important. While this explanation is appropriate for anhydrobiotic organisms, the same is not true of partially desiccated organisms, which tend not to vitrify. At 30°C, heat tolerance was improved in partially desiccated larvae of B. antarctica following pre-exposure to 0, 75 and 98% RH (Benoit et al. 2009). In this instance, the up-regulation of heat shock proteins and the accumulation of trehalose were suggested as being possible explanations for the enhanced heat tolerance by overcompensating for any opposing injuries.
The heat tolerance of E. murphyi has been little explored, except in a study by Everatt et al. (2014), which showed larval survival up to 39°C for 1 h. In the current study, larvae of E. murphyi showed 100% survival up to 35°C and also survived temperatures as high as 39°C for the longer period of 2 h (Fig. 4). Larvae of B. antarctica are likewise able to survive temperatures above 30°C (Benoit et al. 2009). Although these organisms rarely, if ever, experience temperatures nearing 30 or even 25°C, their heat tolerance is not surprising. A number of other studies, including those by Deere et al. (2006), Everatt, Convey et al. (2013), Sinclair et al. (2006) and Slabber et al. (2007) have similarly shown appreciable heat tolerance in polar invertebrates. Such findings are consistent with the “thermal sensitivity hypothesis,” which states that the thermal sensitivity of invertebrates to a temperature rise declines with increasing latitude (Addo-Bediako et al. 2000; Deutsch et al. 2008).
Conclusion
The Arctic and Antarctic are similar in that they include both cold and arid landscapes. However, the Diptera of these regions, based on the evidence presented here, have not adapted similarly. Two strategies of living in a dry environment have been identified. The Arctic dipteran Heleomyza borealis utilizes desiccation resistance, while the Antarctic dipteran Eretmoptera murphyi principally uses desiccation tolerance. Divergence between Antarctic and Arctic invertebrates has also been shown between Belgica antarctica and Megaphorura arctica, which utilize distinct molecular mechanisms in response to desiccation (Teets et al. 2012). Desiccation was found to induce cross-tolerance to low temperatures, but not high temperatures, in E. murphyi. An ability to acclimate in this way would likely be beneficial in the variable climates typical of polar terrestrial habitats, where low temperature and low water availability are commonly encountered simultaneously.
Acknowledgements
MJE was funded by the Natural Environment Research Council (RRBN15266) and was supported by the British Antarctic Survey and the University of Birmingham. PC is supported through Natural Environment Research Council core funding to the British Antarctic Survey’s Ecosystems programme. This paper also contributes to the Evolution and Biodiversity in Antarctica research programme of the Scientific Committee on Antarctic Research.
References
Addo-Bediako
A.,
Chown
S.L.
&
Gaston
K.J. 2000.
Thermal tolerance, climatic variability and latitude. Proceedings of the Royal Society of London B 267,
739–745.
Publisher Full Text
Allegrucci
G.,
Carchini
G.,
Convey
P.
&
Sbordoni
V. 2012.
Evolutionary geographic relationships among orthocladine chironomid midges from maritime Antarctic and sub-Antarctic islands. Biological Journal of the Linnaean Society 106,
258–274.
Publisher Full Text
Allegrucci
G.,
Carchini
G.,
Todisco
V.,
Convey
P.
&
Sbordoni
V. 2006.
A molecular phylogeny of Antarctic Chironomidae and its implications for biogeographical history. Polar Biology 29,
320–326.
Publisher Full Text
Bahrndorff
S.,
Petersen
S.O.,
Loeschcke
V.,
Overgaard
J.
&
Holmstrup
M. 2007.
Differences in cold and drought tolerance of High Arctic and sub-Arctic populations of Megaphorura arctica Tullberg 1876 (Onychiuridae: Collembola). Cryobiology 55,
315–323.
PubMed Abstract | Publisher Full Text
Bahrndorff
S.,
Tunnacliffe
A.,
Wise
M.J.,
McGee
B.,
Holmstrup
M.
&
Loeschcke
V. 2009.
Bioinformatics and protein expression analyses implicate LEA proteins in the drought response of Collembola. Journal of Insect Physiology 55,
210–217.
PubMed Abstract | Publisher Full Text
Bayley
M.
&
Holmstrup
M. 1999.
Water vapor absorption in arthropods by accumulation of myoinositol and glucose. Science 285,
1909–1911.
PubMed Abstract | Publisher Full Text
Bayley
M.,
Petersen
S.O.,
Knigge
T.,
Köhler
H.-R.
&
Holmstrup
M. 2001.
Drought acclimation confers cold tolerance in the soil collembolan Folsomia candida. Journal of Insect Physiology 47,
1197–1204.
PubMed Abstract | Publisher Full Text
Benoit
J.B.,
Lopez-Martinez
G.,
Elnitsky
M.A.,
Lee
R.E.
&
Denlinger
D.L. 2009.
Dehydration-induced cross tolerance of Belgica antarctica larvae to cold and heat is facilitated by trehalose accumulation. Comparative Biochemistry and Physiology A 152,
518–523.
Publisher Full Text
Benoit
J.B.,
Lopez-Martinez
G.,
Michaud
M.R.,
Elnitsky
M.A.,
Lee
R.E.
&
Denlinger
D.L. 2007.
Mechanisms to reduce dehydration stress in larvae of the Antarctic midge, Belgica antarctica. Journal of Insect Physiology 53,
656–667.
PubMed Abstract | Publisher Full Text
Benoit
J.B.,
Yoder
J.A.,
Lopez-Martinez
G.,
Elnitsky
M.A.,
Lee
R.E.
&
Denlinger
D.L. 2007.
Adaptations for the maintenance of water balance by three species of Antarctic mites. Polar Biology 31,
539–547.
Publisher Full Text
Block
W.,
Lewis Smith
R.I.
&
Kennedy
A.D. 2009.
Strategies of survival and resource exploitation in the Antarctic fellfield ecosystem. Biological Reviews of the Cambridge Philosophical Society 84,
449–484.
PubMed Abstract | Publisher Full Text
Cannon
R.J.
&
Block
W. 1988.
Cold tolerance of microarthropods. Biological Reviews of the Cambridge Philosophical Society 63,
23–77.
Publisher Full Text
Clarke
A.,
Morris
G.J.,
Fonseca
F.,
Murray
B.J.,
Acton
E.
&
Price
H.C. 2013.
A low temperature limit for life on Earth. PLoS One 8,
e66207.
PubMed Abstract | PubMed Central Full Text | Publisher Full Text
Convey
P. 1992.
Aspects of the biology of the midge, Eretmoptera murphyi Schaeffer (Diptera: Chironomidae), introduced to Signy Island, maritime Antarctic. Polar Biology 12,
653–657.
Publisher Full Text
Convey
P. 1996.
The influence of environmental characteristics on life history attributes of Antarctic terrestrial biota. Biological Reviews 71,
191–225.
Publisher Full Text
Convey
P.,
Bindschadler
R.,
di Prisco
G.,
Fahrbach
E.,
Gutt
J.,
Hodgson
D.A.,
Mayewski
P.A.,
Summerhayes
C.P.
&
Turner
J. 2009.
Antarctic climate change and the environment. Antarctic Science 21,
541–563.
Publisher Full Text
Convey
P.
&
Block
W. 1996.
Antarctic Diptera: ecology, physiology and distribution. European Journal of Entomology 93,
1–13.
Convey
P.,
Block
W.
&
Peat
H.J. 2003.
Soil arthropods as indicators of water stress in Antarctic terrestrial habitats? Global Change Biology 9,
718–730.
Publisher Full Text
Crowe
J.H.
&
Madin
K.A.C. 1975.
Anhydrobiosis in nematodes: evaporative water loss and survival. Journal of Experimental Zoology 193,
323–334.
Publisher Full Text
Danks
H. 2000.
Dehydration in dormant insects. Journal of Insect Physiology 46,
837–852.
PubMed Abstract | Publisher Full Text
Deere
J.A.,
Sinclair
B.J.,
Marshall
D.J.
&
Chown
S.L. 2006.
Phenotypic plasticity of thermal tolerances in five oribatid mite species from sub-Antarctic Marion Island. Journal of Insect Physiology 52,
693–700.
PubMed Abstract | Publisher Full Text
Deutsch
C.A.,
Tewksbury
J.J.,
Huey
R.B.,
Sheldon
K.S.,
Ghalambor
C.K.,
Haak
D.C.
&
Martin
P.R. 2008.
Impacts of climate warming on terrestrial ectotherms across latitude thermal safety margin. Proceedings of the National Academy of Sciences of the United States of America 105,
6668–6672.
PubMed Abstract | PubMed Central Full Text | Publisher Full Text
Elnitsky
M.A.,
Hayward
S.A.L.,
Rinehart
J.P.,
Denlinger
D.L.
&
Lee
R.E. 2008.
Cryoprotective dehydration and the resistance to inoculative freezing in the Antarctic midge, Belgica antarctica. The Journal of Experimental Biology 211,
524–530.
PubMed Abstract | Publisher Full Text
Everatt
M.J.,
Convey
P.,
Worland
M.R.,
Bale
J.S.
&
Hayward
S.A.L. 2013.
Heat tolerance and physiological plasticity in the Antarctic collembolan, Cryptopygus antarcticus, and mite, Alaskozetes antarcticus. Journal of Thermal Biology 38,
264–271.
Publisher Full Text
Everatt
M.J.,
Convey
P.,
Worland
M.R.,
Bale
J.S.
&
Hayward
S.A.L.
2014. Are the Antarctic dipteran, Eretmoptera murphyi, and Arctic collembolan, Megaphorura arctica, vulnerable to rising temperatures? Bulletin of Entomological Research.
Everatt
M.J.,
Worland
M.R.,
Bale
J.S.,
Convey
P.
&
Hayward
S.A.L. 2012.
Pre-adapted to the maritime Antarctic? Rapid cold hardening of the midge, Eretmoptera murphyi. Journal of Insect Physiology 58,
1104–1111.
PubMed Abstract | Publisher Full Text
Everatt
M.J.,
Worland
M.R.,
Convey
P.,
Bale
J.S.
&
Hayward
S.A.L. 2013.
The impact of salinity on survival and temperature tolerance of the Antarctic collembolan, Cryptopygus antarcticus. Physiological Entomology 38,
202–210.
Publisher Full Text
Gibbs
A.G.,
Chippindale
A.K.
&
Rose
M.R. 1997.
Physiological mechanisms of evolved desiccation resistance in Drosophila melanogaster. The Journal of Experimental Biology 200,
1821–1832.
PubMed Abstract
Hawes
T.C.,
Couldridge
C.E.,
Bale
J.S.,
Worland
M.R.
&
Convey
P. 2006.
Habitat temperature and the temporal scaling of cold hardening in the High Arctic collembolan, Hypogastrura tullbergi (Schäffer). Ecological Entomology 31,
450–459.
Publisher Full Text
Hayward
S.A.L.,
Rinehart
J.P.,
Sandro
L.H.,
Lee
R.E.
&
Denlinger
D.L. 2007.
Slow dehydration promotes desiccation and freeze tolerance in the Antarctic midge Belgica antarctica. The Journal of Experimental Biology 210,
836–844.
PubMed Abstract | Publisher Full Text
Hazel
J.R. 1995.
Thermal adaptation in biological membranes: is homeoviscous adaptation the explanation? Annual Review of Physiology 57,
19–42.
PubMed Abstract | Publisher Full Text
Hengherr
S.,
Heyer
A.G.,
Köhler
H.-R.
&
Schill
R.O. 2008.
Trehalose and anhydrobiosis in tardigrades—evidence for divergence in responses to dehydration. The FEBS Journal 275,
281–288.
PubMed Abstract | Publisher Full Text
Hengherr
S.,
Reuner
A.,
Brümmer
F.
&
Schill
R.O. 2010.
Ice crystallization and freeze tolerance in embryonic stages of the tardigrade Milnesium tardigradum. Comparative Biochemistry and Physiology A 156,
151–155.
Publisher Full Text
Hengherr
S.,
Worland
M.R.,
Reuner
A.,
Brümmer
F.
&
Schill
R.O. 2009.
Freeze tolerance, supercooling points and ice formation: comparative studies on the subzero temperature survival of limno-terrestrial tardigrades. The Journal of Experimental Biology 212,
802–807.
PubMed Abstract | Publisher Full Text
Hinton
H.E. 1951.
A new chironomid from Africa, the larvae of which can be dehydrated without injury. Proceedings of the Zoological Society (Calcutta) 121,
371–380.
Publisher Full Text
Hinton
H.E. 1960.
Cryptobiosis in the larva of Polypedilum vanderplanki Hint (Chironomidae). Journal of Insect Physiology 5,
286–300.
Publisher Full Text
Holmstrup
M.,
Hedlund
K.
&
Boriss
H. 2002.
Drought acclimation and lipid composition in Folsomia candida: implications for cold shock, heat shock and acute desiccation stress. Journal of Insect Physiology 48,
961–970.
PubMed Abstract | Publisher Full Text
Holmstrup
M.
&
Zachariassen
K.E. 1996.
Physiology of cold hardiness in earthworms. Comparative Biochemistry and Physiology A 115,
91–101.
Publisher Full Text
Hughes
K.A.
&
Worland
M.R. 2010.
Spatial distribution, habitat preference and colonization status of two alien terrestrial invertebrate species in Antarctica. Antarctic Science 22,
221–231.
Publisher Full Text
Kennedy
A.D. 1993.
Water as a limiting factor in the Antarctic terrestrial environment: a biogeographical synthesis. Arctic Antarctic and Alpine Research 25,
308–315.
Publisher Full Text
Lee
R.E.,
Chen
C.P.
&
Denlinger
D.L. 1987.
A rapid cold-hardening process in insects. Science 238,
1415–1417.
PubMed Abstract | Publisher Full Text
Lee
R.E.,
Elnitsky
M.A.,
Rinehart
J.P.,
Hayward
S.A.L.,
Sandro
L.H.
&
Denlinger
D.L. 2006.
Rapid cold-hardening increases the freezing tolerance of the Antarctic midge Belgica antarctica. The Journal of Experimental Biology 209,
399–406.
PubMed Abstract | Publisher Full Text
Levis
N.A.,
Yi
S.
&
Lee
R.E. 2012.
Mild desiccation rapidly increases freeze tolerance of the goldenrod gall fly, Eurosta solidaginis: evidence for drought-induced rapid cold-hardening. The Journal of Experimental Biology 215,
3768–3773.
PubMed Abstract | Publisher Full Text
Li
A.,
Benoit
J.B.,
Lopez-Martinez
G.,
Elnitsky
M.A.,
Lee
R.E.
&
Denlinger
D.L. 2009.
Distinct contractile and cytoskeletal protein patterns in the Antarctic midge are elicited by desiccation and rehydration. Proteomics 9,
2788–2798.
PubMed Abstract | Publisher Full Text
Lopez-Martinez
G.,
Benoit
J.B.,
Rinehart
J.P.,
Elnitsky
M.A.,
Lee
R.E.
&
Denlinger
D.L. 2009.
Dehydration, rehydration, and overhydration alter patterns of gene expression in the Antarctic midge, Belgica antarctica. Journal of Comparative Physiology B 179,
481–491.
Publisher Full Text
Lopez-Martinez
G.,
Elnitsky
M.A.,
Benoit
J.B.,
Lee
R.E.
&
Denlinger
D.L. 2008.
High resistance to oxidative damage in the Antarctic midge Belgica antarctica, and developmentally linked expression of genes encoding superoxide dismutase, catalase and heat shock proteins. Insect Biochemistry and Molecular Biology 38,
796–804.
PubMed Abstract | Publisher Full Text
Michaud
M.R.,
Benoit
J.B.,
Lopez-Martinez
G.,
Elnitsky
M.A.,
Lee
R.E.
&
Denlinger
D.L. 2008.
Metabolomics reveals unique and shared metabolic changes in response to heat shock, freezing, and desiccation in the Antarctic midge, Belgica antarctica. Journal of Insect Physiology 54,
645–655.
Publisher Full Text
Parkash
R.,
Chahal
J.,
Sharma
V.
&
Dev
K. 2012.
Adaptive associations between total body color dimorphism and climatic stress related traits in a stenothermal circumtropical Drosophila species. Insect Science 19,
247–262.
Publisher Full Text
Parkash
R.,
Rajpurohit
S.
&
Ramniwas
S. 2008.
Changes in body melanisation and desiccation resistance in highland vs. lowland populations of D. melanogaster. Journal of Insect Physiology 54,
1050–1056.
PubMed Abstract | Publisher Full Text
Parkash
R.,
Ramniwas
S.,
Rajpurohit
S.
&
Sharma
V. 2008.
Variations in body melanisation impact desiccation resistance in Drosophila immigrans in western Himalayas. Journal of Zoology 276,
219–227.
Publisher Full Text
Parkash
R.,
Sharma
V.
&
Kalra
B. 2009.
Impact of body melanisation on desiccation resistance in montane populations of D. melanogaster: analysis of seasonal variation. Journal of Insect Physiology 55,
898–908.
PubMed Abstract | Publisher Full Text
Pedersen
P.G.
&
Holmstrup
M. 2003.
Freeze or dehydrate: only two options for the survival of subzero temperatures in the Arctic enchytraeid Fridericia ratzeli. Journal of Comparative Physiology B 173,
601–609.
Publisher Full Text
Popovic
Z.D.,
Purac
J.,
Kojic
D.,
Pamer
E.,
Worland
M.R.,
Blagojevic
D.P.
&
Grubor-Lajsic
G. 2011.
Lea protein expression during cold-induced dehydration in the Arctic collembola Megaphorura arctica. Archives of Biological Sciences 63,
681–683.
Publisher Full Text
Ramløv
H.
&
Westh
P. 1992.
Survival of the cryobiotic eutardigrade Adorybiotus coronifer during cooling to −196°C: effect of cooling rate, trehalose level and short term acclimation. Cryobiology 19,
125–130.
Ramniwas
S.,
Kajla
B.,
Dev
K.
&
Parkash
R. 2013.
Direct and correlated responses to laboratory selection for body melanisation in Drosophila melanogaster: support for the melanisation-desiccation resistance hypothesis. The Journal of Experimental Biology 216,
1244–1254.
PubMed Abstract | Publisher Full Text
Ring
R.
&
Danks
H. 1998.
The role of trehalose in cold-hardiness and desiccation. Cryo-letters 19,
275–282.
Sakurai
M.,
Furuki
T.,
Akao
K.,
Tanaka
D.,
Nakahara
Y.,
Kikawada
T.,
Watanabe
M.
&
Okuda
T. 2008.
Vitrification is essential for anhydrobiosis in an African chironomid, Polypedilum vanderplanki. Proceedings of the National Academy of Sciences of the United States of America 105,
5093–5098.
PubMed Abstract | PubMed Central Full Text | Publisher Full Text
Shuker
K.P.N.
2001. The hidden powers of animals: uncovering the secrets of nature. London: Marshall Editions.
Sinclair
B.J.,
Terblanche
J.S.,
Scott
M.B.,
Blatch
G.L.,
Jaco Klok
C.
&
Chown
S.L. 2006.
Environmental physiology of three species of Collembola at Cape Hallett, North Victoria Land, Antarctica. Journal of Insect Physiology 52,
29–50.
PubMed Abstract | Publisher Full Text
Slabber
S.,
Worland
M.R.,
Leinaas
H.P.
&
Chown
S.L. 2007.
Acclimation effects on thermal tolerances of springtails from sub-Antarctic Marion Island: indigenous and invasive species. Journal of Insect Physiology 53,
113–125.
PubMed Abstract | Publisher Full Text
Smith
T.,
Wharton
D.A.
&
Marshal
C.J. 2008.
Cold tolerance of an Antarctic nematode that survives intracellular freezing: comparisons with other nematode species. Journal of Comparative Physiology B 178,
93–100.
Publisher Full Text
Sømme
L.
&
Block
W. 1982.
Cold hardiness of Collembola at Signy Island, maritime Antarctic. Oikos 38,
168–176.
Publisher Full Text
Sømme
L.
&
Meier
T. 1995.
Cold hardiness of Tardigrada from Dronning Maud Land, Antarctica. Polar Biology 15,
221–224.
Sørensen
J.G.
&
Holmstrup
M. 2011.
Cryoprotective dehydration is widespread in Arctic springtails. Journal of Insect Physiology 57,
1147–1153.
Publisher Full Text
Speight
M.R.,
Hunter
M.D.
&
Watt
A.D. 2008. Insects and climate. In
M.R.
Speight
et al. (eds.): Ecology of insects: concepts and applications. 2nd edn. Pp. 33–60.
Hoboken,
NJ: Wiley-Blackwell.
Strathdee
A.T.
&
Bale
J.S. 1998.
Life on the edge: insect ecology in Arctic environments. Annual Review of Entomology 43,
85–106.
PubMed Abstract | Publisher Full Text
Teets
N.M.,
Peyton
J.T.,
Colinet
H.,
Renault
D.,
Kelley
J.L.,
Kawarasaki
Y.,
Lee
R.E.
&
Denlinger
D.L. 2012.
Gene expression changes governing extreme dehydration tolerance in an Antarctic insect. Proceedings of the National Academy of Sciences of the United States of America 109,
20744–20749.
PubMed Abstract | PubMed Central Full Text | Publisher Full Text
Timmermans
M.J.T.N.,
Roelofs
D.,
Nota
B.,
Ylstra
B.
&
Holmstrup
M. 2009.
Sugar sweet springtails: on the transcriptional response of Folsomia candida (Collembola) to desiccation stress. Insect Molecular Biology 18,
737–746.
PubMed Abstract | Publisher Full Text
Treonis
A.M.
&
Wall
D.H. 2005.
Soil nematodes and desiccation survival in the extreme arid environment of the Antarctic Dry Valleys. Integrative and Comparative Biology 45,
741–750.
PubMed Abstract | Publisher Full Text
Turner
J.,
Bindschadler
R.,
Convey
P.,
Di Prisco
G.,
Fahrbach
E.,
Gutt
J.,
Hodgson
D.A.,
Mayewski
P.A.
&
Summerhayes
C.P. (eds.) 2009. Antarctic climate change and the environment.
Cambridge: Scientific Committee for Antarctic Research, 554 Pp.
Watanabe
M.,
Kikawada
T.,
Minagawa
N.,
Yukuhiro
F.
&
Okuda
T. 2002.
Mechanism allowing an insect to survive complete dehydration and extreme temperatures. Journal of Experimental Biology 205,
2799–2802.
PubMed Abstract
Wharton
D.A. 2003.
The environmental physiology of Antarctic terrestrial nematodes: a review. Journal of Comparative Physiology B 173,
621–628.
Publisher Full Text
Wharton
D.A. 2011.
Anhydrobiosis: the model worm as the model? Current Biology 21,
R578–R579.
PubMed Abstract | Publisher Full Text
Wharton
D.A.
&
Barclay
S. 1993.
Anhydrobiosis in the free-living Antarctic nematode Panagrolaimus davidi. Fundamental and Applied Nematology 16,
17–22.
Worland
M.R. 2010. Eretmoptera murphyi: pre-adapted to survive a colder climate. Physiological Entomology 29,
127–137.
Worland
M.R.
&
Block
W. 1999.
Ice-nucleating bacteria from the guts of two sub-Antarctic beetles, Hydromedion sparsutum and Perimylops antarcticus (Perimylopidae). Cryobiology 38,
60–67.
PubMed Abstract | Publisher Full Text
Worland
M.R.,
Block
W.
&
Grubor-Lajsic
G.O. 2000.
Survival of Heleomyza borealis (Diptera, Heleomyzidae) larvae down to −60°C. Physiological Entomology 25,
1–5.
Publisher Full Text
Worland
M.R.,
Grubor-Lajsic
G.
&
Montiel
P. 1998.
Partial desiccation induced by sub-zero temperatures as a component of the survival strategy of the Arctic collembolan Onychiurus arcticus (Tullberg). Journal of Insect Physiology 44,
211–219.
PubMed Abstract | Publisher Full Text
Worland
M.R.,
Leinaas
H.P.
&
Chown
S.L. 2006.
Supercooling point frequency distributions in Collembola are affected by moulting. Functional Ecology 20,
323–329.
Publisher Full Text