RESEARCH/REVIEW ARTICLE
Deterrent activities in the crude lipophilic fractions of Antarctic benthic organisms: chemical defences against keystone predators
Laura Núñez-Pons & Conxita Avila
Department of Animal Biology (Invertebrates) & Biodiversity Research Institute, Faculty of Biology, University of Barcelona, Av. Diagonal 643, ES-08028 Barcelona, Catalonia, Spain
Abstract
Generalist predation constitutes a driving force for the evolution of chemical defences. In the Antarctic benthos, asteroids and omnivore amphipods are keystone opportunistic predators. Sessile organisms are therefore expected to develop defensive mechanisms mainly against such consumers. However, the different habits characterizing each predator may promote variable responses in prey. Feeding-deterrence experiments were performed with the circumpolar asteroid macropredator Odontaster validus to evaluate the presence of defences within the apolar lipophilic fraction of Antarctic invertebrates and macroalgae. A total of 51% of the extracts were repellent, yielding a proportion of 17 defended species out of the 31 assessed. These results are compared with a previous study in which the same fractions were offered to the abundant circum-Antarctic amphipod Cheirimedon femoratus. Overall, less deterrence was reported towards asteroids (51%) than against amphipods (80.8%), principally in sponge and algal extracts. Generalist amphipods, which establish casual host–prey sedentary associations with biosubstrata (preferentially sponges and macroalgae), may exert more localized predation pressure than sea stars on certain sessile prey, which would partly explain these results. The nutritional quality of prey may interact with feeding deterrents, whose production is presumed to be metabolically expensive. Although optimal defence theory posits that chemical defences are managed and distributed as to guarantee protection at the lowest cost, we found that only a few organisms localized feeding deterrents towards most exposed and/or valuable body regions. Lipophilic defensive metabolites are broadly produced in Antarctic communities to deter opportunistic predators, although several species combine different defensive traits.
Keywords
Antarctic invertebrates; Antarctic algae; chemical ecology; sea star Odontaster validus; amphipod Cheirimedon femoratus; chemical defence.
Correspondence
Laura Núñez-Pons, Department of Animal Biology (Invertebrates) & Biodiversity Research Institute, Faculty of Biology, University of Barcelona, Av. Diagonal 643, ES-08028 Barcelona, Catalonia, Spain.
E-mail: lauguau@gmail.com
(Published: 7 April 2014)
Polar Research 2014. © 2014 L. Núñez-Pons & C. Avila. This is an Open Access article distributed under the terms of the Creative Commons Attribution-Noncommercial 3.0 Unported License (http://creativecommons.org/licenses/by-nc/3.0/), permitting all non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Citation: Polar Research 2014, 33, 21624, http://dx.doi.org/10.3402/polar.v33.21624
Predation constitutes an interaction in which an organism attacks another, resulting in its eventual digestion. In prey with clonal growth, predation normally occurs only on a part of the victim, rarely invoking death (Heck & Valentine 1995; Sánchez et al. 2004). After sub-lethal attacks, this type of prey may respond with plastic anti-predatory behaviour, morphology or chemistry, or a combination (Harvell 1984; Vermeij 1994). One efficient weapon against predation is chemical defence. Many natural substances that display allelochemical (signalling) roles have no known primary metabolic function. These are considered secondary metabolites and are presumably costly but essential for fitness. Anti-feedant chemical defences have been described in marine organisms, such as phlorotannins and terpene alcohols in algae, alkaloids in poriferans and dithiocarbamates in hydrozoans, which decrease the vulnerability of susceptible prey to future attacks (Cronin & Hay 1996; Lindquist 2002; Thoms et al. 2007; Toth et al. 2007). Chemical defences are an investment, as organisms must divert energy otherwise assigned to growth or reproduction and its cost is compensated by greater survival potential (Harvell 1990). Several theories have been formulated to explain where energy is saved and the costs of chemical defence minimized. The optimality theory (OT) proposes the use of all-purpose defensive tactics against a variety of enemies (Herms & Mattson 1992). The optimal defence theory (ODT) postulates that deterrent metabolites are directed to particular types of predator and that they work together with other defensive traits to assure survival. According to the ODT, defensive chemicals are allocated to the most valuable or vulnerable tissues (Rhoades & Gates 1976).
The mechanisms by which defensive metabolites promote predation avoidance are still unknown since many are not highly poisonous but instead seem to have an effect promoting bad taste (Paul 1992; McClintock & Baker 2001). Nutritional quality must also be taken into consideration since some repellents are more (or only) effective in low-quality foods. This is likely because nutrients may bind to deterrent molecules or compete with these for enzymes, masking the stimuli that elicit rejection (Duffy & Paul 1992; Cruz-Rivera & Hay 2003). Hence, highly nutritive potential prey likely require larger amounts of, or more potent, defences to avoid consumption, while prey items of lower nutrient content tend to develop weaker defensive systems. For this reason, there are also costs for consumers: because they may need energetically expensive detoxification mechanisms when ingesting chemically defended organisms, they often eat poor-quality foods made up of undefended organisms (Hay et al. 1987; Lindquist & Hay 1995; Cruz-Rivera & Hay 2003). By consuming small amounts of a variety of foods, predators may dilute the possible negative effects derived from ingesting defensive metabolites, while also accruing the nutritional benefits derived from a mixed diet (Bernays et al. 1994; Stachowicz et al. 2007; Sotka et al. 2009). As generalist predators are more prevalent, defences tend to be developed against them as opposed to specialists (Paul et al. 2007).
Antarctic sea floors are characterized by unpredictable food availability, driving most consumers to develop flexible opportunistic foraging strategies. There is a predominantly circumpolar distribution of many of the dominant benthic organisms including keystone macroinvertebrate predators, mainly asteroids (Dayton et al. 1974; McClintock 1994). Populations of amphipods with diversified diets are found in extremely high densities in association with biosubstrata, which often represent their potential prey as well as their shelter (see De Broyer et al. 2007 for a review). In this case, small sedentary consumers could constitute a potential threat, sometimes worse than larger wandering predators, such as echinoderms or fish (Hay et al. 1987; McClintock & Baker 2001; Toth et al. 2007).
Sea stars feed by extruding the cardiac stomach against their food items (Sloan 1980; Brusca & Brusca 2003). Hence, in Antarctic waters, where sea stars are keystone predators (McClintock 1994), ODT would predict that defences would be concentrated primarily in the outermost body parts of prey. However, it should be noted that other effective consumers of smaller size, like small crustaceans, may promote different types of defence distribution, especially in prey with large body perforations (i.e., oscula), like hexactinellid sponges that may be attacked from the inside (Peters et al. 2009).
In Antarctic marine organisms, measures to deter consumption have been well documented by research using sea stars as model consumers; however, post-ingestion repulsive reactions have been scarcely tested (for reviews see Avila et al. 2008; McClintock et al. 2010; Taboada et al. 2012). The current study evaluates the palatability of Antarctic benthic invertebrates and algae with respect to the sympatric asteroid predator, Odontaster validus. Our past research has focused on the ecological role of lipidic metabolites in Southern Ocean organisms, in part because known feeding deterrents in marine environments are mostly lipid-soluble (Sotka et al. 2009). In this study, we sought to evaluate defensive strategies developed against a common consumer—O. validus—in Antarctic waters by using the lipophilic fractions of selected organisms to determine the presence of apolar defences and the hypothetical within-body allocation of deterrents. The results are discussed and compared with previous experiments with another important predator, the amphipod Cheirimedon femoratus.
Materials and methods
Field collection of Antarctic benthic samples
Benthic invertebrates and algae were collected during four Antarctic cruises: two in the Eastern Weddell Sea on board the RV Polarstern (ANT XV/3 and ANT XXI/2 cruises); a third one on board the RV BIO Hespérides around the South Shetland Islands (ECOQUIM-2 cruise); and the fourth at Deception Island (ACTIQUIM-1 campaign). Sampling took place at a total of 24 stations between 0 and 1524 m depth by using bottom and Agassiz trawls (AGTs) and an epibenthic sledge (ES) and, at shallow sites, by scuba diving (SD). At collection, all invertebrate and macroalgal samples exhibited a healthy, fresh appearance; none of them showed signs of decomposition. Specimens belonging to the same species and from the same collecting station were grouped as a single sample, representing the mean population of each site. Fresh organisms were then photographed on deck, and a representative portion of each sample was fixed in 10% formalin solution for further taxonomical identification at the University of Barcelona. The rest of the material was subsequently frozen at −20°C and shipped back to our laboratory, where it was preserved until processed.
Processing of samples: dissections and chemical extraction procedures
Invertebrate samples with large enough volumes to allow dissection were divided into different body parts in order to determine the potential chemical defences present in these different parts. Samples were dissected as follows: sponges were divided into internal/external and apical/basal regions; pennatulacean cnidarians into poliparium/axe/peduncule; and ascidians into external/internal parts. All whole and dissected invertebrate samples and algae were then chemically extracted with acetone using a pestle at room temperature. The resulting extract was fractioned in diethyl ether and afterwards in butanol, always evaporating the solvent in vacuo using a rotary evaporator. All steps were repeated three times, except for the butanol fractionation, which was done only once. At the end, we obtained a dry ether and a dry butanol fraction and an aqueous residue. The dry ether and butanol fractions were weighted and divided into the total dry weight of the corresponding sample, as follows: DW (total dry weight)=EE (ether extract)+BE (butanol extract)+DR (dry extracted residue). This yielded extracts at the natural sample concentration: N=EE/DW (Table 1). See Núñez-Pons et al. (2012) for detailed information regarding sample collection and extraction yields.
Table 1 Data from the Antarctic benthic organisms analysed. The second column shows the natural concentration values (%) of diethyl ether extracts (EE) in each sample, obtained by dividing EE weight by the total dry weight.
| Taxonomic group and species namea
|
% NEE in dry weightb
|
Location |
Gearc
|
Depth (m) |
| Algae |
| Ochrophyta |
|
|
|
|
| Adenocystis utricularis (Bory de Saint-Vincent) Skottsberg 1907
|
5.87 |
Snow Is. |
SD |
1.5 |
| Ascoseira mirabilis Skottsberg 1907 |
2.79 |
Livingston Is. |
SD |
0.7 |
| Desmarestia anceps Montagne 1842 |
7.63 |
Deception Is. |
SD |
7.5 |
| Desmarestia antarcticad Moe & Silva 1989 epiphyted by Geminocarpus austrogeorgiae Skottsberg, 1907 |
4.49 |
Livingston Is. |
SD |
0.7 |
| Desmarestia menziesiie J. Agardh 1848 |
1.35 |
Deception Is. |
AGT |
109.7e
|
| Rhodophyta |
|
|
|
|
| Georgiella confluens (Reisch) Kylin 1956 |
0.97 |
Livingston Is. |
SD |
0.7 |
| Gigartina skottsbergii Setchell & Gardner, 1936 |
0.25 |
Deception Is. |
SD |
12 |
| Palmaria decipiens (Reinsch) Ricker 1987 |
0.66 |
Deception Is. |
SD |
1.3 |
| Porifera |
| Demospongiae |
|
|
|
|
| Isodictya toxophila Burton, 1932 |
API: 5.28; BAS: 6.79 |
Weddell Sea |
BT |
332.8 |
| Hexactinellida |
|
|
|
|
| Anoxycalyx (Scolymastra) joubini Topsent, 1916 (1) |
API: 1.29; BAS: 0.73 |
Weddell Sea |
AGT |
175.2 |
| Anoxycalyx (Scolymastra) joubini Topsent, 1916 (2) |
EXT: 2.60; INT: 1.83 |
Weddell Sea |
BT |
290.8 |
| Rossella fibulata Schulze & Kirkpatrick, 1910 |
EXT: 1.19; INT: 1.75 |
Weddell Sea |
BT |
332.8 |
| Rossella nuda Topsent, 1901 |
1.17 |
Weddell Sea |
BT |
308.8 |
| Rossella vanhoffeni (Schulze & Kirkpatrick, 1910) |
API: 2.36; BAS: 0.61 |
Weddell Sea |
ES |
882 |
| Rossella villosa Burton, 1929 |
API: 1.05; BAS: 0.99 |
Weddell Sea |
AGT |
288.0 |
| Rossella sp.1 Carter, 1872 |
EXT: 1.20; INT: 1.08 |
Weddell Sea |
AGT |
288.0 |
| Cnidaria |
| Anthozoa |
|
|
|
|
| Alcyonium antarcticum Wright & Studer, 1889 |
1.97 |
Weddell Sea |
BT |
337.2 |
| Alcyonium haddoni Wright & Studer, 1889 |
4.61 |
Deception Is. |
SD |
9 |
| Alcyonium roseum van Ofwegen, Häussermann & Försterra, 2007 |
3.21 |
Weddell Sea |
AGT |
416 |
| Primnoisis antarctica (Studer, 1878) (1) |
0.45 |
Weddell Sea |
BT |
294.8 |
| Primnoisis antarctica (Studer, 1878) (2) |
0.58 |
Weddell Sea |
BT |
294.8 |
| Thouarella laxa Versluys, 1906 (1) |
1.44 |
Weddell Sea |
BT |
308.8 |
| Thouarella laxa Versluys, 1906 (2) |
1.27 |
Weddell Sea |
BT |
290.8 |
| Thouarella laxa Versluys, 1906 (3) |
0.88 |
Weddell Sea |
BT |
294.8 |
| Thouarella laxa Versluys, 1906 (4) |
1.44 |
Deception Is. |
AGT |
100.4 |
| Thouarella minuta Zapata-Guardiola & López-González 2009 |
2.00 |
Weddell Sea |
BT |
338 |
| Umbellula antarctica Kükenthal and Broch, 1911 |
POL: 11.59; AX: 1.08 |
Weddell Sea |
BT |
338 |
| Hydrozoa |
| Staurotheca antarctica Hartlaub, 1904 |
3.93 |
Weddell Sea |
BT |
597.6 |
| Symplectoscyphus glacialis (Haderholm 1904) |
1.02 |
Weddell Sea |
AGT |
175.2 |
| Chordata (Ascidiacea) |
| Aplidium falklandicum Millar, 1960 |
4.20 |
Weddell Sea |
BT |
332.8 |
| Aplidium fuegiense Cunningham, 1871 |
7.71 |
Weddell Sea |
AGT |
228.4 |
| Aplidium meridianum (Sluiter, 1906) |
12.85 |
Weddell Sea |
BT |
284.4 |
| Synoicum adareanum (B&W) (Herdman, 1902) (1) |
EXT: 2.00; INT: 3.31 |
Weddell Sea |
BT |
337.2 |
| Synoicum adareanum (B&W) (Herdman, 1902) (2) |
API: 5.57; EXT: 1.81; INT: 2.73 |
Weddell Sea |
AGT |
288.0 |
| Synoicum adareanum (Br) (Herdman, 1902) |
3.68 |
Weddell Sea |
AGT |
277.2 |
| Synoicum adareanum (O) (Herdman, 1902) (1) |
2.04 |
Weddell Sea |
AGT |
288.0 |
| Synoicum adareanum (O) (Herdman, 1902) (2) |
EXT: 2.80; INT: 2.64 |
Weddell Sea |
BT |
337.2 |
| Bryozoa |
| Isoschizoporella secunda Hayward and Taylor, 1984 |
0.75 |
Weddell Sea |
AGT |
277.2 |
| Echinodermata (Holoturoidea) |
| Peniagone vignioni Herouard, 1901 |
2.19 |
Weddell Sea |
AGT |
1524.8 |
| Hemichordata (Pterobranchia) |
| Cephalodiscus nigrescens Lankester, 1905 |
7.43 |
Weddell Sea |
BT |
284.4 |
| aMorphotypes are abbreviated as follows: black and white (B&W); brown (Br); orange (O). |
| bBody parts are abbreviated as follows: apical (API); basal (BAS); external (EXT); INT: internal; poliparium (POL); axis (AX). |
| cCollecting gear is abbreviated as follows: bottom trawl (BT); Agassiz trawl (AGT); epibenthic sledge (ES); scuba diving in shallow sites (SD). |
| dDesmarestia antarctica samples were second-year plants. |
| eFor D. menziesii, depth record is that given by the dredge collection station, but sample algal specimens probably come from shallower bottoms; material was in healthy condition. Unlikely to have drifted to the site. |
Feeding-deterrence bioassays with sea stars
Individuals of the Antarctic asteroid Odontaster validus, measuring between 6 and 10.5 cm diameter, were collected at different sites in Port Foster Bay, Deception Island, South Shetland Islands, Antarctica (62° 59.369' S, 60° 33.424' W), for feeding-repellence assays during the ACTIQUIM-1 (December–February 2008/09) and ACTIQUIM-2 (January 2010) cruises by SD from 3 to 15 m depth. Once testing was over, the sea stars were returned to the sea.
Odontaster validus is a predator commonly used in feeding acceptability studies (for review see Avila et al. 2008). This asteroid is readily available, and its feeding response lends itself quite well to laboratory bioassays. The collected sea stars were kept in large tanks of seawater at Spain's Gabriel de Castilla Station on Deception Island and were deprived of food for 5 days. The methodology followed in the experiments is detailed elsewhere (Avila et al. 2000; Iken et al. 2002). In brief, the assays consisted of 10 replicates, each with a 2.5 L container filled with seawater and one sea star, which was presented with a shrimp feeding cube sufficiently small (5×5×5 mm; 13.09±3.43 mg of dry mass) to be fully consumed by the asteroid. These tiny shrimp food items contained 12.4% protein, 9.1% carbohydrates and 1.5% lipids, and 17.8 kJ g−1 in dry wt and 4.1 kJ g−1 wet wt, according to the manufacturer's nutritional information and the Atwater factor system (Atwater & Benedict 1902). They were loaded either with lipophilic extracts from Antarctic invertebrates and algae, applied at their respective natural concentration in the treatment test, or with solvent carrier alone (diethyl ether) in the control tests. In both cases, the solvent was left to totally evaporate under a flow hood. The factor to normalize tissue concentrations of each fraction (hereafter referred to as natural concentration) was calculated on a dry-weight basis employing the total dry weight of each sample, as previously mentioned. Dry weight was chosen, rather than volume or wet weight, because it eliminates the water content, which may entail notable deviations in aquatic porous samples (Table 1). When the sea stars everted the cardiac stomach and bolted down whole food cubes, we could measure the “defence per shrimp cube.” After 24 h, the food items that had been eaten were noted, and feeding repellence was evaluated by applying Fisher's exact tests for each assay referred to the control run simultaneously (Sokal & Rohlf 1995; Fig. 1). Uneaten extract-treated shrimp pieces were preserved frozen for further extraction and analysis by thin layer chromatography to check for possible alterations in the extracts. No changes were observed. Because EEs are not hydrophilic and the water temperature was fairly cold (ca. −1°C), little, if any, loss to the water column was expected.
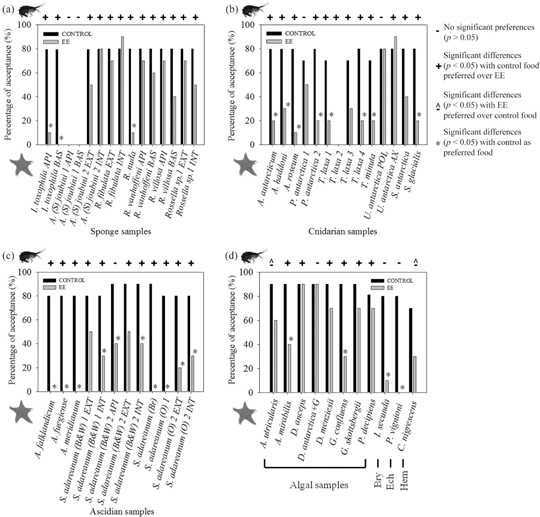
Fig. 1 Bar diagrams of the feeding repellence bioassays with the sea star Odontaster validus for the four major groups assessed: (a) sponges, (b) cnidarians, (c) ascidians, (d) algae + minor groups (abbreviated as follows: Bryozoa [Bry], Echinodermata [Ech], Hemichordata [Hem]), showing the results of each paired test with control and extract-treated diets (EE), represented by the percentage of acceptance. Asterisks indicate significant differences with the control as the preferred food (Fisher's exact test). The hexactinellid S. (A.) joubini sample 1 and the octocoral Thouarella laxa sample 2 were not assayed in this experiment. At the top of each graph the results are compared (Exact Wilcoxon test) with findings from feeding preference bioassays in a parallel study with the amphipod Cheirimedon femoratus (Núñez-Pons et al. 2012).
Results
A total of 31 species comprising 40 samples of Antarctic sponges (eight), cnidarians (13), ascidians (eight), bryozoans (one), echinoderms (one), hemichordate pterobranchs (one) and macroalgae (eight) yielded 52 lipophilic extracts, which were tested in feeding acceptability assays against the Antarctic macropredator sea star Odontaster validus. The control assays revealed a minimum consumption rate of seven pieces of shrimp out of 10 in all tests. Significant repellent activities towards O. validus were detected in 17 species out of the 31 assessed. Of the 49
fractions tested, 25 indicated the presence of deterrents (51%). The remaining 14 species provided 24 extracts (49%) that were suitable for sea star consumption. In terms of groups, ascidians and cnidarians exhibited the highest activity with 83.3 and 61.5% of repellent extracts, respectively, followed by algae (25%) and sponges (23.1%; Fig. 2). The only bryozoan tested, as well as the holoturian samples were also significantly repellent, whereas the hemichordate pterobranch yielded an inactive ether fraction. In the group of the ascidians, both Synoicum adareanum (black and white morphotype [B&W]) samples 1 and 2 with internal fractions were rejected by O. validus, but their external lipophilic extracts were consumed (elicited no significant feeding deterrent activity). Most seaweed and poriferan fractions were suitable for sea star consumption, except for two algal extracts from Ascoseira mirabilis and Georgiella confluens, and two sponge fractions from Isodictya toxophyla and Rossella nuda, which were rejected. Finally, five cnidarian extracts, including those from the polyparium and axis body regions of Umbellula antarctica, two from samples of the gorgonians Primnoisis antarctica and Thouarella laxa, and one from the hydrozoan Staurotheca antarctica were accepted by the star (Table 1; Fig. 1).
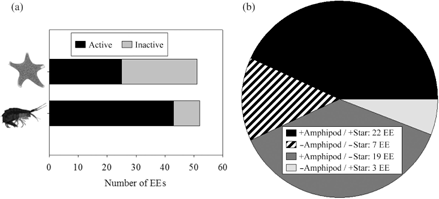
Fig. 2
Comparison of results from bioassays with the sea star Odontaster validus and those with the amphipod Cheirimedon femoratus from Núñez-Pons et al. (2012) testing the incidence of repellent activities in diethyl ether extracts (EEs) from the samples assessed, which included invertebrates and macroalgae. In (a), a horizontal bar diagram shows the total activity of all assessed extracts assessed, contrasting tests with asteroids and amphipods. In (b) coincident and non-coincident deterrent activities are shown for all the tested fractions, comparing the two experiments; plus signs indicate the presence of feeding repellents and minus signs indicate the lack of them.
Discussion
Feeding deterrence as means of defence against Antarctic benthic predators
The assays with the sea star Odontaster validus demonstrated a widespread presence of lipid-soluble deterrents within cnidarians and ascidians. The opposite pattern is shown in sponges and algae. The low deterrence we found in sponges conflicts with other published findings. Past studies, however, usually tested demosponge species, which are prolific sources of bioactive natural products and are presumably far better defended than the hexactinellids, which represent most of our Porifera (McClintock & Baker 2001; Avila et al. 2008; McClintock et al. 2010; Taboada et al. 2012).
When comparing the current study with parallel work testing the same fractions against the omnivore lyssianasid amphipod Cheirimedon femoratus (Núñez-Pons et al. 2012), we observed that out of the 49 lipophilic extracts assessed in both bioassays, 22 (44.9%) were deterrent and five (10.2%) were suitable for both predators. Therefore, 55.1% (27 fractions) elicited the same response in the two predators. Twenty-two fractions had different results for the two species, 19 (38.8%) being discouraging for the amphipod but palatable for O. validus, and the remaining three fractions were repellent just for the sea star. Overall, there was a 32.7% higher incidence of deterrency towards amphipod feeding than against sea star ingestion, especially in the groups of sponges and algae (Figs. 1, 2).
Even if O. validus and C. femoratus have both circumpolar-eurybathic distributions, and extensive generalist diets (scavenger, detritivore, planktivore; Bregazzi 1972; McClintock 1994; De Broyer et al. 2007), the distinct ecological habits of these predators may promote variable defensive responses in potential prey. In general, sea stars are mobile macropredators that start extra-oral digestion from the surface of the victims (Sloan 1980). Amphipods instead feed with minute peripheral bites, and only prolonged feeding would allow access to internal tissues. However, in prey organisms with evident openings, these small crustaceans may reach the inner regions to forage (Peters et al. 2009). We compare the results of the present study with those from Núñez-Pons et al. (2012), estimating prey protective adaptations against two different predators.
Methodological considerations and feeding deterrent activities
The experiments with Odontaster validus were no-choice feeding repellence tests, whereas those performed with Cheirimedon femoratus by Núñez-Pons et al. (2012) were preference assays with-choice. When allowed to choose between extract-treated versus palatable control food pearls, predators may be better able to discriminate repellent agents. Another difference in the two studies is that the diets were distinct in energetic content: alginate pearls used with the amphipod C. femoratus were less nutritious (19 kJ g−1 dry wt and 1.5 kJ g−1 wet wt) than the shrimp cubes presented to O. validus (17.8 kJ g−1 dry wt and 4.1 kJ g−1 wet wt; by Atwater factors; Atwater & Benedict 1902). Therefore, some repellent extracts might have been less evident in the sea star tests due to a higher nutritional value of shrimp compared to alginate pearls (and potentially compared to some of the samples too). Despite these modifications, we are still able to make comparative approximations related to deterrence potential since nutritious foods can interact with defensive metabolites and constrain the types or concentrations that might be efficacious in nature for discouraging predators. An idea of the potency of deterrents can be drawn by measuring their activity in the two kinds of experiments using assay foods of distinct energy content (Duffy & Paul 1992; Cruz-Rivera & Hay 2003). Most of the fractions deterrent to amphipods but accepted by sea stars came from less nutritious samples: hexactinellid sponges; and fibrous body parts: tunics of S. adareanum (B&W) and the stalk from U. antarctica. In these cases, moderate to poor chemical defence along with low nutritional value may operate in tandem to make these body parts less appealing. In general, the optimal assay foods are those energetically equivalent to the studied prey; however, O. validus would not feed on gelified diets (authors’ pers. obs.). This study tested only ether fractions, and possible defences exclusive to other fractions were not analysed.
Macroalgae and sponges as potential prey or as potential host-and-prey
Lipophilic deterrence in algae was shown to be low towards sea stars and high towards amphipods. This could be due, in part, to a higher ingestion of algal material by C. femoratus ovigerous females and juveniles during summer (Bregazzi 1972). Hydrophilic deterrents (i.e., phlorotanins), often contributing to asteroid repellence in algae, could have been missed in our analysis (Amsler et al. 2005).
Antarctic benthic amphipods generally associate with living substrata with no obligate relationships (De Broyer et al. 2001), obtaining tri-dimensional habitat and food as well as chemical refuges from prospective enemies, like fish, when hosts are chemically defended (McClintock et al. 2009; Zamzow et al. 2010). Cheirimedon femoratus may be found as deep as 1500 m (Krapp et al. 2008). At Deception Island, it is both abundant and commonly found on macrophytes and poriferans (authors’ pers. obs.), similar to other northern Cheirimedon amphipods (Vader 1984). Such a niche is filled in some Antarctic areas by several other species (Huang et al. 2007; Amsler et al. 2009). Organism associations adapt in diverse interactions depending on the chemical potential of the host and the feeding adaptations of the grazers. As part of its opportunistic habits, C. femoratus may graze on host tissues directly (authors’ pers. obs.), while also profiting from adhered detritus and diatoms (Bregazzi 1972; Graeve et al. 2001). This leads to a more constant localized pressure on host-and-prey organisms than that exerted by wandering macropredators (Hay et al. 1987; Toth et al. 2007), like O. validus, which forages on ubiquitous prey with less recurrent encounters (McClintock 1987, 1994).
Our algae are comprised of Antarctic brown and red seaweed, which are rich sources of food (11–13 kJ g−1 dry wt; Montgomery & Gerking 1980; Gomez & Westermeier 1995) and bioactive molecules. Except for one species, all sponges were hexactinellids with no reported secondary metabolites (Blunt et al. 2013 and previous reports). Their high spicule content and low energetic value (5–6 kJ g−1 dry wt; McClintock 1987; Barthel 1995) are believed to discourage predators. Average ether-lipophilic yields showed low values in hexactinellids (ca. 1.3%) and in Rodophyta (0.6%), and higher ones (ca. 4.4%) in brown algae (Table 1). The small ether fraction yield in red algae may reflect their small lipid content (Montgomery & Gerking 1980). Antarctic poriferans and macroalgae hold high diversities of amphipods (Kunzmann 1996; Huang et al. 2007; Amsler et al. 2009). Moreover, some algae act as a chemical refuges, including Desmarestia menziesii with lipophilic deterrents and D. anceps with hydrophilic defences; this might have been missed in our study, which only tested ether partitions. Our inactive extract of D. menziesii from deeper waters (ca. 110 m) may have different chemistry due to the variation in depths or the extraction protocol, which yielded unexpected inactivity. Other algae, like Palmaria decipiens, are more preferred as food (though not for our amphipod) but are not preferred as hosts (Amsler et al. 2005; Aumack et al. 2010; Bucolo et al. 2011). Analogous chemical refuges have not been described in Antarctic sponges, yet some defended species host dense amphipod populations (Amsler et al. 2009). Most of the seaweed and sponge fractions reflected no activity against O. validus, but were rejected by C. femoratus, which may use them as substrata and occasionally as prey. The few fractions that repelled both asteroids and amphipods were those from the macroalgae Ascoseira mirabilis and Georgiella confluens, the demosponge Isodictya toxophila and the hexactinellid Rossella nuda. Rossella nuda is abundant and primarily foraged by spongivorous generalists, including O. validus. The richer fractions and repellent chemistry of I. toxophila supports the greater bioactivity of demosponges compared to glass sponges (McClintock 1987; Barthel 1995). Extracts that showed palatability in this study and also towards C. femoratus in our previous study were those from the algae Adenocystis utricularis and Desmarestia antarctica (second-year) epiphyted by Geminocarpus austrogeorgiae (Table 1, Fig. 1). Amsler et al. (2005) proposed that in second-year D. antarctica mild acidity could assist weak hydrophilic repellents against sea stars, while in first-year algae the lipophilic extract was already effective, suggesting a loss of lipophilic defences with senescence. Glass sponges are readily preyed upon by Odontaster and Acanthaster sea stars (McClintock 1987), and as it turns out, no lipophilic repellents or external defence allocation were shown to assist these sponges in asteroid avoidance (Furrow et al. 2003). Instead, efficient defences towards amphipods were found distributed throughout the volcano-shaped rossellids. Opportunistic amphipods, through lax associations, may influence the chemical ecology of macroalgae and sponges and change the expectations of the ODT (Rhoades & Gates 1976), replacing, in some cases, asteroids as the main inducers of defence distribution.
Anti-predatory protection in other invertebrate groups
Antarctic Cnidaria and Ascidiacea are rich in bioactive metabolites (Blunt et al. 2013 and previous reports) and feeding repellents (Koplovitz et al. 2009; Núñez-Pons et al. 2010). Our cnidarian samples comprised hydrozoans and anthozoans whereas the ascidians included colonial species. Soft corals and colonial ascidians are highly energetic (16 and 15 kJ g−1 dry wt, respectively; Slattery & McClintock 1995; McClintock et al. 2004), while hydroids and gorgonians are also quite nutritious (Coma et al. 1998). Ether fraction yields may illustrate this outcome: ascidians ca. 4.9%; soft corals ca. 3.3%; gorgonians ca. 1.2% (Table 1). Antarctic cnidarians and ascidians represent transitory biosubstrata for amphipods in shelf communities not dominated by sponges or algae (Dayton et al. 1974; De Broyer et al. 2001). In the present study, most samples were chemically defended, but other tactics might co-occur in these groups.
Hydrozoans use lipophilic as well as nematocyst-based defences even if considered redundant by the OT (Herms & Mattson 1992; Stachowicz & Lindquist 2000; Lindquist 2002). Staurotheca antarctica yielded a rich fraction deterrent to amphipods but inactive against sea stars. Nematocysts may protect against asteroids, which seem particularly vulnerable when stinging happens on sensitive ambulacral feet or cardiac stomach mucosa, and Syntheciidae hydroids are generally armoured with penetrating cnidos, often injecting polar proteinaceous venoms (Shostak 1995; Ostman 2000). Thus, S. antarctica, in using repellent lipids against amphipods and nematocyst against asteroids, might contravene the OT. Nonetheless, polar fractions may contain other defences not tested here. The hydroid Symplectoscyphus glacialis (Family Sertulariidae) rarely presents penetrating nematocysts (Shostak 1995), and its extract was significantly deterrent to both predators. Regarding Anthozoa, Umbellula antarctica is a pennatulacean with a fibrous axis and a distal crown of giant (3–4 cm long) polyps (Pasternak 1962; Dolan 2008). Both regions revealed distinct bioactivities and thin layer chromatography profiles, suggesting that lipid repellents were present only in the axis. The chemically unprotected and exposed polyparium is presumed to either rely on hydrophilic repellents and/or on nematocysts for defence, which actively participate in capturing prey (Dolan 2008). Autozooid nematocysts in the pennatulid sea pansy, Renilla kollekeri, keep sea star predators away in a similar way (Kastendiek 1976). The fibrous stalk of U. antarctica, denuded of nematocysts, did possess deterrents for amphipods. Since alternative defences seem to occur in different body regions, OT and ODT could both be sustained for U. antarctica. Soft corals and gorgonians generally lack stinging nematocysts and likely rely on chemical defence (Sammarco & Coll 1992), as reflected in our results. Only one fraction from Primnoisis antarctica sample 1 was accepted by both consumers, and one extract from Thouarella laxa was only suitable for the star. Both fraction yields were less abundant than active conspecific extracts (Table 1; Fig. 1).
Many colonial ascidians protect their common external tunic with alternative tactics other than organic deterrents. For example, the Antarctic species Distaplia cylindrica and D. colligans combine lipidic defences and inorganic chemistry (acid, heavy metals; McClintock et al. 2004; Koplovitz et al. 2009). None of the species studied here showed a similar kind of defence (Koplovitz et al. 2009; Lebar et al. 2011). Moreover, tunics may be nutritiously unattractive (McClintock et al. 1991; Pisut & Pawlik 2002). Among our Synoicum adareanum samples, as is common in this species (Varela 2007), there were three colour morphs; black and white (B&W), brown (Br), and orange (O) morphotypes. The dissected O sample exhibited no defence allocation. In contrast, the internal extracts of both B&W samples caused strong rejection by O. validus and
C. femoratus, while the external colonial tunic fractions were accepted by sea stars. B&W morph tunics were thick and tough and yielded poorer lipophilic fractions than their inner regions (Table 1, Fig. 1). To ward off predators, the B&W samples rely on lipophilic distasteful metabolites in the inner tissues. Smaller quantities of these deterrents present in the external tunic may work together with low nutritional value to discourage external asteroid attack. The participation of polar products, again, cannot be ruled out. Finally, the rich apical fraction from a B&W sample containing siphon mouths and common cloaca, was rejected only by the sea star. The allocation of deterrent activities prevalent in internal regions of B&W S. adareanum samples might provide support for the ODT. Colonial ascidians invest much energy for reproduction and tend to produce complex, chemically-defended larvae (Lambert 2005). External damages due to eventual predation attacks rarely kill colonies but instead cause reversible injuries on the outer protective common tunic and death of some clonal zooids. Hence, storing repellents in inner tissues (gonads), which, in this case, are more vital for species survival than the outer colonial tunic, is consistent with the ODT (Rhoades & Gates 1976).
In less represented taxa, the abundant extract from the hemichordate pterobranch displayed no repellency in any assay. These animals may not need chemical protection since they live hidden from major enemies inside the coenoecia, colonial encasements hardened with agglutinated foreign material (Ridewood 1911; Brusca & Brusca 2003). Again, the existence of polar deterrents cannot be discarded. In contrast, the bryozoan and holothurian fractions reflected the presence of feeding deterrents against O. validus. The calcified bryozoan Isoschizoporella secunda afforded poor extract yields. It harbours sessile “trap-door” avicularia that are proposed to function as active mechanical deterrents to zooid-level predators (amphipods, nematodes, polychaetes) and as secretion sites for bioactive compounds (Carter et al. 2010). Hence, this branched bryozoan could avoid amphipod attacks through entrapments of appendages by avicularia and rely on chemistry against asteroids (Bryan et al. 1998). Finally, the elpidiid holothurian Peniagone vignioni, with an ability to swim (Wigham et al. 2008), may escape from many bottom-dwelling consumers. Thus, repellents against sea stars would only be useful while the animal is feeding on the substrate surface (Table 1; Fig. 1).
Concluding remarks
Antarctic benthic ecosystems are considered stable but adapted to marked seasonalities of nutrient supply and composed of many defended sessile species with long life-spans subjected to intense generalist predation (Dayton et al. 1974; Avila et al. 2008). Most defensive deterrents do not totally prevent attacks, but they reduce the attractiveness of the organism compared to other coexisting prey, while generalist feeding mitigates possible toxicities of secondary metabolites and compensates for poor-quality diets (Bernays et al. 1994; Stachowicz et al. 2007; Sotka et al. 2009). Combined effects of nutrient content with defensive chemicals are not appreciable prior to ingestion and may affect predators differently. In sea stars, gustation likely takes place with the cardiac stomach or the ambulacral system (Sloan 1980; Kidawa 2005), whereas scavenging lysianassoid amphipods have well-developed gustatory gnathopods (Kaufmann 1994). Amphipods seem particularly sensitive to lipidic deterrents (Duffy & Paul 1992; Cruz-Rivera & Hay 2003), as we have observed in Cheirimedon femoratus (Núñez-Pons et al. 2012). Accordingly, macroalgae and invertebrates mostly yield lipid-soluble repellents (Sotka et al. 2009). Our investigations have aimed at improving our understanding of the ecological importance of lipophilic metabolites in Antarctic organisms, which require sizable lipid reserves. We are, however, aware of the limitations of testing only EEs in terms of the relative difference among consumers and how these can interact with distinct metabolite types. Further studies in progress will analyse other fractions. Differences in extraction protocols, bioassay methodologies (actual ingestion versus chemo-sensitive reactions in sea stars) and experimental predatory amphipod species, as well as the distance between collection sites, make it difficult to combine our results with those of other Antarctic studies in order to draw strong conclusions (for reviews see Avila et al. 2008; McClintock et al. 2010). Further studies should seek to fill in the gaps of understanding Antarctic chemical ecology.
Acknowledgements
We thank M. Rodríguez-Arias, J. Vázquez, B. Figuerola, F.J. Cristobo and S. Taboada for their invaluable help during the Antarctic cruises. Comments from the reviewers were very helpful, as well as the English refinements of M. Keinath. Thanks are due to the crew of the RV Polarstern. The Marine Technology Unit (UTM; under the Spanish National Research Council [CSIC]), the crew of the RV Las Palmas and the staff of Gabriel de Castilla Station gave logistic support. We are thankful to A. Gómez-Garreta, A. Ribera-Siguán, P. Ríos, M. Varela and A. Bosch for taxonomical assistance. Funding was provided by the Ministry of Science and Innovation of Spain (projects CGL2004-03356/ANT, CGL2007-65453/ANT and CGL2010-17415/ANT).
References
Amsler
C.D.,
Iken
K.,
McClintock
J.B.,
Amsler
M.O.,
Peters
K.J.,
Hubbard
J.M.,
Furrow
F.B.
&
Baker
B.J. 2005.
Comprehensive evaluation of the palatability and chemical defenses of subtidal macroalgae from the Antarctic Peninsula. Marine Ecology Progress Series 294,
141–159.
Publisher Full Text
Amsler
M.O.,
McClintock
J.B.,
Amsler
C.D.,
Angus
R.A.
&
Baker
B.J. 2009.
An evaluation of sponge-associated amphipods from the Antarctic Peninsula. Antarctic Science 21,
579–589.
Publisher Full Text
Atwater
W.O.
&
Benedict
F.G. 1902. Experiments on the metabolism of matter and energy in the human body, 1898–1900. Washington, DC: Government Printing Office.
Aumack
C.F.,
Amsler
C.D.,
McClintock
J.B.
&
Baker
B.J. 2010.
Chemically mediated resistance to mesoherbivory in finely branched macroalgae along the western Antarctic Peninsula. European Journal of Phycology 45,
19–26.
Publisher Full Text
Avila
C.,
Iken
K.,
Fontana
A.
&
Gimino
G. 2000.
Chemical ecology of the Antarctic nudibranch Bathydoris hodgsoni Eliot, 1907: defensive role and origin of its natural products. Journal of Experimental Biology and Ecology 252,
27–44.
Publisher Full Text
Avila
C.,
Taboada
S.
&
Núñez-Pons
L. 2008.
Marine Antarctic chemical ecology: what is next? Marine Ecology 29,
1–70.
Publisher Full Text
Barthel
D. 1995.
Tissue composition of sponges from the Weddell Sea, Antarctica—not much meat on the bones. Marine Ecology Progress Series 123,
149–153.
Publisher Full Text
Bernays
E.A.,
Bright
K.L.,
Gonzalez
N.
&
Angel
J. 1994.
Dietary mixing in a generalist herbivore—tests of two hypotheses. Ecology 75,
1997–2006.
Publisher Full Text
Blunt
J.W.,
Copp
B.R.,
Keyzers
R.A.,
Munro
M.H.G.
&
Prinsep
M.R. 2013.
Marine natural products. Natural Products Reports 30,
237–323.
Publisher Full Text
Bregazzi
P.K. 1972.
Life cycles and seasonal movements of Cheirimedon femoratus and Tryphosella kergueleni Crustacea Amphipoda. British Antarctic Survey Bulletin 30,
1–34.
Brusca
R.C.
&
Brusca
G.J. 2003. Invertebrates. 2nd edn. Sunderland, MA: Sinauer Associates.
Bryan
P.J.,
Kreider
J.L.
&
Qian
P.Y. 1998.
Settlement of the serpulid polychaete Hydroides elegans (Haswell) on the arborescent bryozoan Bugula neritina (L.): evidence of a chemically mediated relationship. Journal of Experimental Biology and Ecology 220,
171–190.
Publisher Full Text
Bucolo
P.,
Amsler
C.D.,
McClintock
J.B.
&
Baker
B.J. 2011.
Palatability of the Antarctic rhodophyte Palmaria decipiens (Reinsch) RW Ricker and its endo/epiphyte Elachista antarctica Skottsberg to sympatric amphipods. Journal of Experimental Marine Biology and Ecology 396,
202–206.
Publisher Full Text
Carter
M.C.,
Gordon
D.P.
&
Gardner
J.P.A. 2010.
Polymorphism and vestigiality: comparative anatomy and morphology of bryozoan avicularia. Zoomorphology 129,
195–211.
Publisher Full Text
Coma
R.,
Ribes
M.,
Gili
J.M.
&
Zabala
M. 1998.
An energetic approach to the study of life-history traits of two modular colonial benthic invertebrates. Marine Ecology Progress Series 162,
89–103.
Publisher Full Text
Cronin
G.
&
Hay
M.E. 1996.
Induction of seaweed chemical defenses by amphipod grazing. Ecology 77,
2287–2301.
Publisher Full Text
Cruz-Rivera
E.
&
Hay
M.E. 2003.
Prey nutritional quality interacts with chemical defenses to affect consumer feeding and fitness. Ecological Monographs 73,
483–506.
Dayton
P.K.,
Robillia
G.A.,
Paine
R.T.
&
Dayton
L.B. 1974.
Biological accommodation in benthic community at McMurdo Sound Antarctica. Ecological Monographs 44,
105–128.
Publisher Full Text
De Broyer
C.,
Lowry
J.K.,
Jazdzewski
K.
&
Robert
H. 2007. Catalogue of the Gammaridean and Corophiidean Amphipoda of the Southern Ocean, with distribution and ecological data. Bulletin de l'Institut Royal des Sciences Naturelles de Belgique: Biologie 77, Supplement 1.
Bruxelles: Royal Belgian Institute of Natural Sciences.
De Broyer
C.,
Scailteur
Y.,
Chapelle
G.
&
Rauschert
M. 2001.
Diversity of epibenthic habitats of gammaridean amphipods in the eastern Weddell Sea. Polar Biology 24,
744–753.
Publisher Full Text
Dolan
E. 2008. Phylogenetics, systematics and biogeography of deep-sea Pennatulacea (Anthozoa: Octocorallia). Evidence from molecules and morphology. PhD thesis,
University of Southamptom.
Duffy
J.E.
&
Paul
V.J. 1992.
Prey nutritional quality and the effectiveness of chemical defenses against tropical reef fishes. Oecologia 90,
333–339.
Publisher Full Text
Furrow
F.B.,
Amsler
C.D.,
McClintock
J.B.
&
Baker
B.J. 2003.
Surface sequestration of chemical feeding deterrents in the Antarctic sponge Latrunculia apicalis as an optimal defense against sea star spongivory. Marine Biology 143,
443–449.
Publisher Full Text
Gomez
I.
&
Westermeier
R. 1995.
Energy contents and organic constituents in Antarctic and south Chilean marine brown algae. Polar Biology 15,
597–602.
Publisher Full Text
Graeve
M.,
Dauby
P.
&
Scailteur
Y. 2001.
Combined lipid, fatty acid and digestive tract content analyses: a penetrating approach to estimate feeding modes of Antarctic amphipods. Polar Biology 24,
853–862.
Publisher Full Text
Harvell
C.D. 1984.
Predator-induced defense in a marine bryozoan. Science 224,
1357–1359.
PubMed Abstract | Publisher Full Text
Harvell
C.D. 1990.
The ecology and evolution of inducible defenses. Quaterly Review of Biology 65,
323–340.
Publisher Full Text
Hay
M.E.,
Duffy
J.E.
&
Pfister
C.A. 1987.
Chemical defense against different marine herbivores—are amphipods insect equivalents? Ecology 68,
1567–1580.
Publisher Full Text
Heck
K.L.
&
Valentine
J.F. 1995.
Sea-urchin herbivory—evidence for long-lasting effects in subtropical seagrass meadows. Journal of Experimental Marine Biology and Ecology 189,
205–217.
Publisher Full Text
Herms
D.A.
&
Mattson
W.J. 1992.
The dilemma of plants—to grow or defend. Quaterly Review of Biology 67,
283–335.
Publisher Full Text
Huang
Y.M.,
Amsler
M.O.,
McClintock
J.B.,
Amsler
C.D.
&
Baker
B.J. 2007.
Patterns of gammaridean amphipod abundance and species composition associated with dominant subtidal macroalgae from the western Antarctic Peninsula. Polar Biology 30,
1417–1430.
Publisher Full Text
Iken
K.,
Avila
C.,
Fontana
A.
&
Gavagnin
M. 2002.
Chemical ecology and origin of defensive compounds in the Antarctic nudibranch Austrodoris kerguelenensis (Opisthobranchia: Gastropoda). Marine Biology 141,
101–109.
Publisher Full Text
Kastendiek
J. 1976.
Behavior of the sea pansy Renilla kollikeri Pfeffer (Coelenterata: Pennatulacea) and its influence on the distribution and biological interactions of species. Biological Bulletin 151,
518–537.
Publisher Full Text
Kaufmann
R.S. 1994.
Structure and function of chemoreceptors in scavenging lysianassoid amphipods. Journal of Crustacean Biology 14,
54–71.
Publisher Full Text
Kidawa
A. 2005.
Behavioural and metabolic responses of the Antarctic sea star Odontaster validus to food stimuli of different concentration. Polar Biology 28,
449–455.
Publisher Full Text
Koplovitz
G.,
McClintock
J.B.,
Amsler
C.D.
&
Baker
B.J. 2009.
Palatability and chemical anti-predatory defenses in common ascidians from the Antarctic Peninsula. Aquatic Biology 7,
81–92.
Publisher Full Text
Krapp
R.H.,
Berge
J.,
Flores
H.,
Gulliksen
B.
&
Werner
I. 2008.
Sympagic occurrence of eusirid and lysianassoid amphipods under Antarctic pack ice. Deep-Sea Research Part II 55,
1015–1023.
Publisher Full Text
Kunzmann
K. 1996. Associated fauna of selected sponges (Hexactinellida and Demospongiae) from the Weddell Sea, Antarctica. Reports on Polar Research 210.
Bremerhaven: Alfred Wegener Institute.
Lambert
G. 2005.
Ecology and natural history of the protochordates. Canadian Journal of Zoology 83,
34–50.
Publisher Full Text
Lebar
M.D.,
Luttenton
L.,
McClintock
B.,
Amsler
C.D.
&
Baker
B. 2011.
Accumulation of vanadium, manganese, and nickel in Antarctic tunicates. Polar Biology 34,
587–590.
Publisher Full Text
Lindquist
N. 2002.
Tridentatols D-H, nematocyst metabolites and precursors of the activated chemical defense in the marine hydroid Tridentata marginata (Kirchenpauer 1864). Journal Natural Products 65,
681–684.
Publisher Full Text
Lindquist
N.
&
Hay
M.E. 1995.
Can small rare prey be chemically defended—the case for marine larvae. Ecology 76,
1347–1358.
Publisher Full Text
McClintock
J.B. 1987.
Investigation of the relationship between invertebrate predation and biochemical composition, energy content, spicule armament and toxicity of benthic sponges at McMurdo Sound, Antarctica. Marine Biology 94,
479–487.
Publisher Full Text
McClintock
J.B. 1994.
Trophic biology of Antarctic echinoderms. Marine Ecology Progress Series 111,
191–202.
Publisher Full Text
McClintock
J.B.,
Amsler
C.D.
&
Baker
B. 2010.
Overview of the chemical ecology of benthic marine invertebrates along the western Antarctic Peninsula. Integrative and Comparative Biology 50,
967–980.
PubMed Abstract | Publisher Full Text
McClintock
J.B.,
Amsler
M.O.,
Amsler
C.D.,
Southworth
K.J.,
Petrie
C.
&
Baker
B.J. 2004.
Biochemical composition, energy content and chemical antifeedant and antifoulant defenses of the colonial Antarctic ascidian Distaplia cylindrica. Marine Biology 145,
885–894.
Publisher Full Text
McClintock
J.B.,
Amsler
M.O.,
Koplovitz
G.,
Amsler
C.D.
&
Baker
B.J. 2009.
Observations on an association between the dexaminid amphipod Polycheria Antarctica F. Acanthopoda and its ascidian host Distaplia cylindrica. Journal of Crustacean Biology 29,
605–608.
Publisher Full Text
McClintock
J.B.
&
Baker
B.J. 2001. Marine chemical ecology. Boca Raton, FL: CRC.
McClintock
J.B.,
Heine
J.,
Slattery
M.
&
Weston
J. 1991.
Biochemical and energetic composition, population biology, and chemical defense of the Antarctic ascidian Cnemidocarpa verrucosa lesson. Journal of Experimental Marine Biology and Ecology 147,
163–175.
Publisher Full Text
Montgomery
W.L.
&
Gerking
S.D. 1980.
Marine macroalgae as foods for fishes—an evaluation of potential food quality. Environmental Biology of Fishes 5,
143–153.
Publisher Full Text
Núñez-Pons
L.,
Forestieri
R.,
Nieto
R.M.,
Varela
M.,
Nappo
M.,
Rodríguez
J.,
Jiménez
C.,
Castelluccio
F.,
Carbone
M.,
Ramos-Esplá
A.,
Gavagnin
M.
&
Avila
C. 2010.
Chemical defenses of tunicates of the genus Aplidium from the Weddell Sea (Antarctica). Polar Biology 33,
1319–1329.
Publisher Full Text
Núñez-Pons
L.,
Rodríguez-Arias
M.,
Gómez-Garreta
A.,
Ribera-Siguan
A.
&
Avila
C. 2012.
Feeding deterrency in Antarctic marine organisms: bioassays with the omnivore amphipod Cheirimedon femoratus. Marine Ecology Progress Series 462,
163–174.
Publisher Full Text
Ostman
C. 2000.
A guideline to nematocyst nomenclature and classification, and some notes on the systematic value of nematocysts. Scientia Marina 64,
31–46.
Publisher Full Text
Pasternak
T.A. 1962.
Pennatulids of the genus Umbellula Cuvier from the Antarctic and Subantarctic. Issled Faune Morei 1,
105–128.
Paul
V.J. 1992. Ecological roles of marine natural products. Ithaca, NY: Comstock Publications.
Paul
V.J.,
Arthur
K.E.,
Ritson-Williams
R.,
Ross
C.
&
Sharp
K. 2007.
Chemical defenses: from compounds to communities. Biological Bulletin 213,
226–251.
PubMed Abstract | Publisher Full Text
Peters
K.J.,
Amsler
C.D.,
McClintock
J.B.,
van Soest
R.W.M.
&
Baker
B.J. 2009.
Palatability and chemical defenses of sponges from the western Antarctic Peninsula. Marine Ecology Progress Series 385,
77–85.
Publisher Full Text
Pisut
D.P.
&
Pawlik
J.R. 2002.
Anti-predatory chemical defenses of ascidians: secondary metabolites or inorganic acids? Journal of Experimental Marine Biology and Ecology 270,
203–214.
Publisher Full Text
Rhoades
D.F.
&
Gates
R.G. 1976.
Toward a general theory of plant antiherbivore chemistry. Recent Advances in Phytochemistry 10,
168–213.
Ridewood
W.G. 1911. Cephalodiscus. National Antarctic expedition 1901–1904. Natural history 2. Zoology. London: British Museum.
Sammarco
P.W.
&
Coll
J.C. 1992.
Chemical adaptations in the Octocorallia: evolutionary considerations. Marine Ecology Progress Series 88,
93–104.
Publisher Full Text
Sánchez
J.A.,
Gil
M.F.,
Chasqui
L.H.
&
Alvarado
E.M. 2004.
Grazing dynamics on a Caribbean reef-building coral. Coral Reefs 23,
578–583.
Shostak
S. 1995. Nematocyst database. Accessed on the internet at http://www.pitt.edu/~sshostak/cnidocyst_database/ on 6 March 2014
Slattery
M.
&
McClintock
J.B. 1995.
Population structure and feeding deterrence in three shallow-water Antarctic soft corals. Marine Biology 122,
461–470.
Publisher Full Text
Sloan
N.A. 1980.
Aspects of the feeding biology of asteroids. Oceanography and Marine Biology: An Annual Review 18,
57–124.
Sokal
R.R.
&
Rohlf
F.J. 1995. Biometry: the principles and practice of statistics in biological research. New York: W.H. Freeman and Co.
Sotka
E.E.,
Forbey
J.,
Horn
M.,
Poore
A.G.B.,
Raubenheimer
D.
&
Whalen
K.E. 2009.
The emerging role of pharmacology in understanding consumer–prey interactions in marine and freshwater systems. Integrative and Comparative Biology 49,
291–313.
PubMed Abstract | Publisher Full Text
Stachowicz
J.J.,
Bruno
J.F.
&
Duffy
J.E. 2007.
Understanding the effects of marine biodiversity on communities and ecosystems. Annual Review of Ecology, Evolution and Systematics 38,
739–766.
Publisher Full Text
Stachowicz
J.J.
&
Lindquist
N. 2000.
Hydroid defenses against predators: the importance of secondary metabolites versus nematocysts. Oecologia 124,
280–288.
Publisher Full Text
Taboada
S.,
Núñez-Pons
L.
&
Avila
C. 2012.
Feeding repellence of Antarctic and sub-Antarctic benthic invertebrates against the omnivorous sea star Odontaster validus Koehler, 1906. Polar Biology 36,
13–25.
Publisher Full Text
Thoms
C.,
Schupp
P.J.,
Custódio
M.R.,
Lôbo-Hajdu
G.,
Hajdu
E.
&
Muricy
G. 2007. Chemical defense strategies in sponges: a review. In
M.R.
Custódio
et al. (eds.): Porifera research: biodiversity, Innovation and sustainability. Pp. 627–637.
Rio de Janeiro: National Museum of Brazil.
Toth
G.B.,
Karlsson
M.
&
Pavia
H. 2007.
Mesoherbivores reduce net growth and induce chemical resistance in natural seaweed populations. Oecologia 152,
245–255.
PubMed Abstract | Publisher Full Text
Vader
W. 1984.
Notes on Norwegian marine Amphipoda 7. Amphipod associates of Geodia sponges in western Norway. Fauna Norvegian Series A 5,
14–16.
Varela M. 2007. Contribución al conocimiento de las ascidias coloniales (Chordata: Tunicata) de la Antártida Occidental y Región Magallánica. (Contribution to the knowledge of colonial ascidians [Chordata: Tunicata] from Occidental Antarctica and Magellan regions.)
PhD thesis, Universidad de Alicante.
Vermeij
G.J. 1994.
The evolutionary interaction among species: selection, escalation, and coevolution, Annual Review of Ecology. Evolution and Systematics 25,
219–236.
Wigham
B.D.,
Galley
E.A.,
Smith
C.R.
&
Tyler
P.A. 2008.
Inter-annual variability and potential for selectivity in the diets of deep-water Antarctic echinoderms. Deep-Sea Research Part II 55,
2478–2490.
Publisher Full Text
Zamzow
J.P.,
Amsler
C.D.,
McClintock
J.B.
&
Baker
B.J. 2010.
Habitat choice and predator avoidance by Antarctic amphipods: the roles of algal chemistry and morphology. Marine Ecology Progress Series 400,
155–163.
Publisher Full Text