RESEARCH NOTE
Complete breeding failures in ivory gull following unusual rainy storms in North Greenland
Glenn Yannic,1,2 Adrian Aebischer,2,3 Brigitte Sabard2 & Olivier Gilg2,4
1Département de Biologie et Centre d’Études Nordiques, Université Laval, 1045 avenue de la Médecine, Québec G1V 0A6, Canada
2Groupe de Recherche en Ecologie Arctique, 16 rue de Vernot, FR-21440 Francheville, France
3Museum of Natural History, Chemin du Musée 6, CH-1700 Fribourg, Switzerland
4Laboratoire Biogéosciences, Unité Mixte de Recherche Centre National de la Recherche Scientifique 5561, Université de Bourgogne, 6 Boulevard Gabriel, FR-21000 Dijon, France
Abstract
Natural catastrophic events such as heavy rainfall and windstorms may induce drastic decreases in breeding success of animal populations. We report the impacts of summer rainfalls on the reproductive success of ivory gull (Pagophila eburnea) in north-east Greenland. On two occasions, at Amdrup Land in July 2009 and at Station Nord in July 2011, we observed massive ivory gull breeding failures following violent rainfall and windstorms that hit the colonies. In each colony, all of the breeding birds abandoned their eggs or chicks during the storm. Juvenile mortality was close to 100% at Amdrup Land in 2009 and 100% at Station Nord in 2011. Our results show that strong winds associated with heavy rain directly affected the reproductive success of some Arctic bird species. Such extreme weather events may become more common with climate change and represent a new potential factor affecting ivory gull breeding success in the High Arctic.
Keywords
Pagophila eburnea; breeding failure; Greenland; endangered species; summer precipitation; climate change.
Correspondence
Glenn Yannic, Département de Biologie et Centre d’Études Nordiques, Université Laval, 1045 avenue de la Médecine, Québec G1V 0A6, Canada. E-mail: glenn.yannic@gmail.com
(Published: 13 March 2014)
Polar Research 2014. © 2014 G. Yannic et al. This is an Open Access article distributed under the terms of the Creative Commons Attribution-Noncommercial 3.0
Unported License (http://creativecommons.org/licenses/by-nc/3.0/), permitting all non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Citation: Polar Research 2014, 33, 22749, http://dx.doi.org/10.3402/polar.v33.22749
Recent climate change has been especially pronounced in the Arctic, with surface temperatures rising two to four times faster than the global average (Miller et al. 2010) and an accompanying rapid decline of sea ice (Serreze et al. 2007; Stroeve et al. 2007). Both the Arctic warming and sea-ice loss in the past few decades are unprecedented over at least the last few thousand years (Kaufman et al. 2009; Polyak et al. 2010). Climate change is likely to increase the magnitude and frequency of extreme weather events, such as freezing rain or heavy precipitation (Parry et al. 2007), with profound ecological consequences on species that inhabit this biome (Post et al. 2009; Gilg et al. 2012; Post et al. 2013). Arctic vertebrates will be exposed to a host of extreme climatic events away from those in which species evolved. Such events may affect animal populations indirectly, for example, by impacting the distribution and abundance of food resources, or directly, for example, by increasing individual mortality or reducing breeding success (Post et al. 2009; Gilg et al. 2012). Species living in Arctic environments have various behavioural, physiological and morphological adaptations to cope with energetically demanding conditions and are uniquely adapted to survive in the cold and dry summers that characterize the High-Arctic region (Martin & Wiebe 2004; Beaumont et al. 2011). The fraction of Arctic summer precipitation occurring as snow has, however, declined over the past few decades, in unison with an increase in rainfall (Screen & Simmonds 2012). Changes in precipitation regime (snow to rain) during the breeding season may have dramatic consequences on animals, and particularly on juveniles (e.g., Mallory, Gaston, Forbes et al. 2009; Mallory, Gaston & Gilchrist 2009; Pokrovsky et al. 2012; Anctil et al. 2013; Boersma & Rebstock 2014).
Here, we report the impacts of unusual summer violent rainy windstorms on the reproductive success of two ivory gull (Pagophila eburnea) breeding colonies located in north-east Greenland. This species recently received considerable research attention and monitoring effort because breeding populations in Canada have declined by over 80% since the 1980s (Gilchrist & Mallory 2005). It is currently one of the most threatened Arctic bird species for several identified reasons, including biomagnification of contaminants (Braune et al. 2006; Miljeteig et al. 2009; Miljeteig et al. 2012) and reduction of sea ice (Nghiem et al. 2007; Stroeve et al. 2007; Screen & Simmonds 2010), its main habitat (Boertmann et al. 2010; Gilg et al. 2010). Hence, its conservation status has been upgraded to “endangered” in Canada (COSEWIC 2006, uplisted in 2011) and “near threatened” internationally (Birdlife International 2012).
Methods
Study area and species
Two breeding colonies located in the most north-eastern corner of Greenland—at Amdrup Land (80°50′N/14°37′W) and Station Nord (81°35′N/16°39′W)—were monitored (see Gilg et al. 2009 for details on ivory gull breeding sites and numbers in Greenland). The Amdrup Land colony is located on a flat rocky plateau surrounded by glaciers, about 5 km inland. The Station Nord colony is located on a coastal terrace, at about 3 km from a military station, where birds are nesting about 1 km from the year-round ice-covered Arctic Ocean. The sea of this part of Greenland is covered by multiannual drift ice throughout the year, yet localized and predictable bodies of open water, like the North-East Water Polynya, make the region attractive to breeding ivory gulls. The ivory gull usually lays one to two eggs, more rarely three eggs (Mallory et al. 2008). Both parents incubate nests for 24–26 days, eggs typically hatch in mid-July in North Greenland and chicks fledge 30–35 days after hatching.
Data collection
The two colonies were visited during the hatching period. The Amdrup Land colony was visited on four occasions between 25 July and 1 August 2009. The Station Nord colony was visited on two occasions on 4 July and 9 July 2011. For each colony, we monitored the number of occupied nests, laid eggs and hatched chicks. The colonies were not monitored during the storm peak; the breeding failure was assessed by an active survey of the colony the day following the storm. Weather data (temperature and precipitation) were automatically collected by the Danish Meteorological Institute at Station Nord for the period 1961–2011 (Capellen 2012). We used linear regression (for temperature) and general linear model (for precipitation, with a quasi-poisson family model) to examine temporal trends in the data over the past 50 years.
Results
Amdrup Land 2009
A total number of 96 nests were occupied at Amdrup Land during our first visit (25 July 2009; Table 1). The brood size was one (n=49 nests) or two (n=41 nests). For six nests, we were unable to assign already highly mobile chicks to a nest. An average of 1.46±0.50 offspring were produced per nest. On 28 July 2009, around 21:00 local time, a rainy storm started and lasted for 18 hours. Twelve hours after the start of the heavy rain at 09:00 on 29 July, a windstorm rose up that lasted for 36 hours. On 31 July 2009, an active survey of the colony found only two live chicks; these were abandoned chicks hidden in stony crevices. Many chick carcasses (n=36) were found in and around nest cups, with for some, apparent signs of pecking from adults on the body. All eggs that could still be found were crushed in or near the nests. While most adults were still present in a large flock near the colony, all 96 nests were unattended. Given the null chances for these two last downy chicks to survive without parental care, breeding failure can confidently be estimated as being 100%.
Table 1 Number of ivory gull nests and brood sizes documented in the two failed colonies.
| |
|
|
|
Before the storm |
After the storm |
| Locality |
Coordinates |
Year |
Number of nests |
Mean brood
size (±SD) |
Eggs |
Nestlings |
Range (min–max) |
Attended eggs |
Nestlings alive |
| Amdrup Land |
81°35′N/16°39′W |
2009 |
96a
|
1.46±0.50 |
83 |
48 |
(1–2) |
0 |
3 |
| Station Nord |
80°50′N/14°37′W |
2011 |
76b
|
1.64±0.52 |
80 |
2 |
(1–3) |
0 |
0 |
| aFive of the 96 nests were already abandoned before we monitored the colony for the first time. Another one has only been monitored after the storm. Statistics are based on 90 nests. |
| bTwenty-six nests have been monitored only after the storm. Statistics are based on 50 nests. |
Station Nord 2011
A total of 50 nests were occupied in the breeding colony on 4 July 2011 (Table 1). The mean clutch size was then 1.64±0.52 offspring (Table 1). From 5 to 8 July 2011, heavy snow and rains hit the region. On 9 July 2011, when visiting the colony for the second time, none of the previously occupied nests were still active. Neither eggs nor chicks were found in the nests and only a few adults were present in the colony. A second group of birds was breeding a little further from the main colony (nest occupancy checked by scope sighting) but nest content was only monitored after the storm. Here again, none of the 26 occupied nests were active.
Temperature and precipitation
For the period 1961–2011, the air over land warmed by 2.75°C for the month of July at Station Nord (coefficient β=0.066, standard error [SE]=0.003; F1,40=68.17, P<0.001; Fig. 1a). For the same period, the five-year moving average annual rainfall recorded indicates a significant increase in the precipitation regime (β=0.007, SE=0.002, F1,40=6.79, P<0.013; Fig. 1b).
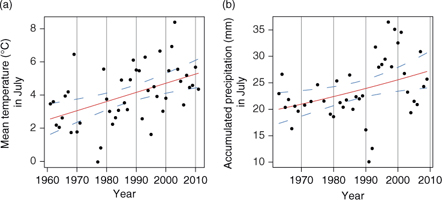
Fig. 1
Long-term changes in temperature and precipitation records at Station Nord. (a) Annual temperature in July from 1961 to 2011 indicates the air over land warmed by 2.75°C in 50 years (coefficient β=0.066, standard error [SE]=0.003; F1,40=68.17, P<0.001). (b) Five-year moving average annual accumulated precipitation in July from 1961 to 2011 (β=0.007, SE=0.002; F1,40=6.79, P=0.013). Weather data were automatically collected by the Danish Meteorological Institute (Capellen 2012). Regression lines and 95% confidence intervals of the predicted models are represented with solid and dashed lines, respectively.
Discussion
The effects of negative and unpredictable events on the reproductive success of birds vary depending on the stage of the reproductive cycle. Unusual and severe weather events such as heavy rainfalls and windstorms can result in the substantial loss of eggs and chicks in Arctic seabirds (Schreiber 2001). At Station Nord, temperatures and precipitation, whether solid or liquid, have been regularly increasing for the past 50 years (Fig. 1). The storms witnessed in 2009 and 2011 occurred during a critical period: egg incubation and brooding of chicks. Adverse weather conditions may also deeply affect adult seabird mortality during the breeding season, as has been shown for northern fulmar (Fulmarus glacialis) and thick-billed murre (Uria lomvia; Mallory, Gaston & Gilchrist 2009). For the two events reported in this study, only juvenile mortality was observed and no adult was found dead (although one satellite tracking tag fitted to an adult for more than two years also suddenly stopped transmitting shortly after the storm in Amdrup Land in 2009, indicating that this adult might have died due to the same event; Gilg unpublished data). Predation after a violent storm can also be facilitated and sometimes appears to be the leading cause of reproductive failure in Arctic seabirds, for example, northern fulmar (Mallory, Gaston, Forbes et al. 2009). Storm-facilitated predation was not observed in our ivory gull colonies, as no predator was directly or indirectly (through observations of tracks or faeces) recorded in the vicinity of the breeding colonies during our monitoring. Intraspecific scavenging, typical in ivory gull (Mallory et al. 2003), is the most likely explanation for the disappearance of some of the lost eggs and chicks.
Although Arctic seabirds are adapted to harsh environmental conditions, unpredictable and extreme environments nonetheless affect their energy balance. Heat losses in cold air, water or in stormy conditions can be extremely high despite adults’ waterproof and well-insulated plumage (Fort et al. 2009). This is magnified for chicks, whose downy plumage is much less waterproof and a poorer insulator than that of the adults. Chicks are therefore highly vulnerable to changes in wind speed and precipitation, with cold rain impacting their energetic metabolism dramatically and potentially resulting in high mortality. In the two reported cases, due to the strength of the observed storm, we suspect that the parents could not properly protect their eggs and young during the storms, as already observed for northern fulmar (Mallory, Gaston & Gilchrist 2009).
Ivory gull in north-east Greenland, and likely elsewhere too, are probably regularly exposed to adverse weather conditions during the breeding season. With the expected increase in extreme weather events in the Arctic (Hassol 2004; Parry et al. 2007), we anticipate that similar breeding failures will occur in the near future due to heavier summer rainfall (Screen & Simmonds 2012), northward range shift being limited for a species whose breeding sites are already restricted to the northernmost lands on Earth. Variability in juvenile survival of long-lived vertebrates can play an important role in population dynamics (Gaillard et al. 1998). Hence, species like the ivory gull are highly vulnerable to changes in the precipitation regime. It remains to be seen whether the ivory gull will be able to withstand, in combination with other challenges, such as pollution and sea-ice decline, new extremes of weather outside its ancestral norms.
Acknowledgements
We are grateful to Vladimir Gilg and Paul Moessiman for their help in the field, to Thomas Broquet, Pierre Legagneux and Emmanuelle Pouivé, who commented and improved a previous draft of the manuscript, and to John Lau Hansen (Greenland Command), the military staff of Station Nord, the Greenland Home Rule, Polog and Norlandair who helped with the permits and the logistics. Funding and equipment were provided by the National Geographic Society, Prix GORE-TEX initiative, Fondation Avenir Finance, the Arctic Ocean Diversity Census of Marine Life Project, Magasins Intermarché, Société Henry Maire, Lestra, MSR, Vitagermine, Moulin des Moines, F. Paulsen and other contributors. GY was supported by grants from foundation Ellis Elliot (Switzerland), Société vaudoise des Sciences naturelles (Switzerland) and Nos Oiseaux (Switzerland). We also thank two anonymous reviewers for their helpful comments.
References
Anctil
A.,
Franke
A.
&
Bêty
J.
2014. Heavy rainfall increases nestling mortality of an Arctic top predator: experimental evidence and long-term trend in peregrine falcons. Oecologia
174, 1033–1043.
PubMed Abstract | PubMed Central Full Text | Publisher Full Text
Beaumont
L.J.,
Pitman
A.,
Perkins
S.,
Zimmermann
N.E.,
Yoccoz
N.G.
&
Thuiller
W. 2011.
Impacts of climate change on the world’s most exceptional ecoregions. Proceedings of the National Academy of Sciences of the United States of America 108,
2306–2311.
PubMed Abstract | PubMed Central Full Text | Publisher Full Text
Birdlife International 2012. Species factsheet: Pagophila eburnea. Accessed on the internet at http://www.birdlife.org on 30 July 2012.
Boersma
P.D.
&
Rebstock
G.A.
2014. Climate change increases reproductive failure in Magellanic penguins. PLoS One
9, e85602, doi: 10.1371/journal.pone.0085602.
Publisher Full Text
Boertmann
D.,
Olsen
K.
&
Gilg
O. 2010.
Ivory gulls breeding on ice. Polar Record 46,
86–88.
Publisher Full Text
Braune
B.M.,
Mallory
M.L.
&
Gilchrist
H.G. 2006.
Elevated mercury levels in declining population of ivory gulls. Marine Pollution Bulletin 52,
969–987.
Publisher Full Text
Capellen
J. 2012. Weather and climate data from Greenland 1958–2011: observation data with description.
Copenhagen: Danish Meteorological Institute.
COSEWIC (Committee on the Status of Endangered Wildlife in Canada) 2006. COSEWIC assessment and update status report on the ivory gull Pagophila eburnea in Canada.
Ottawa: Committee on the Status of Endangered Wildlife in Canada.
Fort
J.,
Porter
W.P.
&
Gremillet
D. 2009.
Thermodynamic modelling predicts energetic bottleneck for seabirds wintering in the northwest Atlantic. Journal of Experimental Biology 212,
2483–2490.
PubMed Abstract | Publisher Full Text
Gaillard
J.-M.,
Festa-Bianchet
M.
&
Yoccoz
N.G. 1998.
Population dynamics of large herbivores: variable recruitment with constant adult survival. Trends in Ecology & Evolution 13,
58–63.
PubMed Abstract | PubMed Central Full Text | Publisher Full Text
Gilchrist
H.G.
&
Mallory
M.L. 2005.
Declines in abundance and distribution of the ivory gull (Pagophila eburnea) in Arctic Canada. Biological Conservation 121,
303–309.
Publisher Full Text
Gilg
O.,
Aars
J.,
Fort
J.,
Gauthier
G.,
Gremillet
D.,
Ims
R.A.,
Kovacs
K.M.,
Meltofte
H.,
Moreau
J.,
Post
E.,
Schmidt
N.M.,
Yannic
G.
&
Bollache
L. 2012.
Climate change and the ecology and evolution of Arctic vertebrates. Annals of the New York Academy of Sciences 1249,
166–190.
PubMed Abstract | Publisher Full Text
Gilg
O.,
Boertmann
D.,
Merkel
F.,
Aebischer
A.
&
Sabard
B. 2009.
Status of the endangered ivory gull, Pagophila eburnea, in Greenland. Polar Biology 32,
1275–1286.
Publisher Full Text
Gilg
O.,
Strøm
H.,
Aebischer
A.,
Gavrilo
M.V.,
Volkov
A.,
Miljeteig
C.
&
Sabard
B. 2010.
Post-breeding movements of the northeast Atlantic ivory gull Pagophila eburnea populations. Journal of Avian Biology 41,
532–542.
Publisher Full Text
Hassol
S.J. 2004. Impacts of a warming Arctic. Arctic climate impact assessment.
Cambridge: Cambridge University Press.
Kaufman
D.S.,
Schneider
D.P.,
McKay
N.P.,
Ammann
C.M.,
Bradley
R.S.,
Briffa
K.R.,
Miller
G.H.,
Otto-Bliesner
B.L.,
Overpeck
J.T.,
Vinther
B.M.
& Arctic Lakes 2K Project Members
2009. Recent warming reverses long-term Arctic cooling. Science
325, 1236–1239.
PubMed Abstract | Publisher Full Text
Mallory
M.L.,
Gaston
A.J.,
Forbes
M.R.
&
Gilchrist
H.G. 2009.
Influence of weather on reproductive success of northern fulmars in the Canadian High Arctic. Polar Biology 32,
529–538.
Publisher Full Text
Mallory
M.L.,
Gaston
A.J.
&
Gilchrist
H.G. 2009.
Sources of breeding season mortality in Canadian Arctic seabirds. Arctic 62,
333–341.
Mallory
M.L.,
Gilchrist
H.G.,
Fontaine
A.J.
&
Akearok
J.A. 2003.
Local ecological knowledge of ivory gull declines in Arctic Canada. Arctic 56,
293–298.
Publisher Full Text
Mallory
M.L.,
Stenhouse
I.J.,
Gilchrist
G.,
Robertson
G.,
Haney
J.C.
&
Macdonald
S.D. 2008. Ivory gull (Pagophila eburnea). In
A.
Poole (ed.):
The birds of North America online.
Ithaca: Cornell Lab of Ornithology. Accessed on the internet at http://bna.birds.cornell.edu/BNA/species/175/ on 11 February 2014.
Martin
K.
&
Wiebe
K.L. 2004.
Coping mechanisms of alpine and Arctic breeding birds: extreme weather and limitations to reproductive resilience. Integrative and Comparative Biology 44,
177–185.
PubMed Abstract | Publisher Full Text
Miljeteig
C.,
Gabrielsen
G.,
Strøm
H.,
Gavrilo
M.,
Lie
E.
&
Jenssen
B. 2012.
Eggshell thinning and decreased concentrations of vitamin E are associated with contaminants in eggs of ivory gulls. Science of the Total Environment 432,
92–99.
Publisher Full Text
Miljeteig
C.,
Strom
H.,
Gavrilo
M.V.,
Volkov
A.,
Jenssen
B.M.
&
Gabrielsen
G.W. 2009.
High levels of contaminants in ivory gull Pagophila eburnea eggs from the Russian and Norwegian Arctic. Environmental Science & Technology 43,
5521–5528.
PubMed Abstract | PubMed Central Full Text | Publisher Full Text
Miller
G.H.,
Alley
R.B.,
Brigham-Grette
J.,
Fitzpatrick
J.J.,
Polyak
L.,
Serreze
M.C.
&
White
J.W.C. 2010.
Arctic amplification: can the past constrain the future? Quaternary Science Reviews 29,
1779–1790.
Publisher Full Text
Nghiem
S.V.,
Rigor
I.G.,
Perovich
D.K.,
Clemente-Colon
P.,
Weatherly
J.W.
&
Neumann
G.
2007. Rapid reduction of Arctic perennial sea ice. Geophysical Research Letters
34, L19504, doi: 10.1029/2007GL031138.
Publisher Full Text
Parry
M.L.,
Canziani
O.F.,
Palutikof
J.P.,
van der Linden
P.J.
&
Hanson
C.E. (eds.):
Climate change 2007. Impacts, adaptation and vulnerability. Contribution of Working Group II to the fourth assessment report of the Intergovernmental Panel on Climate Change. Pp. 653–685.
Cambridge: Cambridge University Press.
Pokrovsky
I.,
Ehrich
D.,
Ims
R.A.,
Kulikova
O.,
Lecomte
N.
&
Yoccoz
N.G.
2012. Assessing the causes of breeding failure among the rough-legged buzzard (Buteo lagopus) during the nestling period. Polar Research
31, article no. 17294, doi: 10.3402/polar.v31i0.17294.
Publisher Full Text
Polyak
L.,
Alley
R.B.,
Andrews
J.T.,
Brigham-Grette
J.,
Cronin
T.M.,
Darby
D.A.,
Dyke
A.S.,
Fitzpatrick
J.J.,
Funder
S.,
Holland
M.,
Jennings
A.E.,
Miller
G.H.,
O’Regan
M.,
Savelle
J.,
Serreze
M.,
St. John
K.,
White
J.W.C.
&
Wolff
E. 2010.
History of sea ice in the Arctic. Quaternary Science Reviews 29,
1757–1778.
Publisher Full Text
Post
E.,
Bhatt
U.S.,
Bitz
C.M.,
Brodie
J.F.,
Fulton
T.L.,
Hebblewhite
M.,
Kerby
J.,
Kutz
S.J.,
Stirling
I.
&
Walker
D.A. 2013.
Ecological consequences of sea-ice decline. Science 341,
519–524.
PubMed Abstract | Publisher Full Text
Post
E.,
Forchhammer
M.C.,
Bret-Harte
M.S.,
Callaghan
T.V.,
Christensen
T.R.,
Elberling
B.,
Fox
A.D.,
Gilg
O.,
Hik
D.S.,
Hoye
T.T.,
Ims
R.A.,
Jeppesen
E.,
Klein
D.R.,
Madsen
J.,
McGuire
A.D.,
Rysgaard
S.,
Schindler
D.E.,
Stirling
I.,
Tamstorf
M.P.,
Tyler
N.J.C.,
van der Wal
R.,
Welker
J.,
Wookey
P.A.,
Schmidt
N.M.
&
Aastrup
P. 2009.
Ecological dynamics across the Arctic associated with recent climate change. Science 325,
1355–1358.
PubMed Abstract | Publisher Full Text
Schreiber
E.A. 2001. Climate and weather effects on seabirds. In
E.A.
Schreiber
&
J.
Burger (eds.): Biology of marine birds. Pp.179–216.
Boca Raton,
FL: CRC Press.
Screen
J.A.
&
Simmonds
I. 2010.
The central role of diminishing sea ice in recent Arctic temperature amplification. Nature 464,
1334–1337.
PubMed Abstract | Publisher Full Text
Screen
J.A.
&
Simmonds
I. 2012.
Declining summer snowfall in the Arctic: causes, impacts and feedbacks. Climate Dynamics 38,
2243–2256.
Publisher Full Text
Serreze
M.,
Holland
M.
&
Stroeve
J. 2007.
Perspectives on the Arctic’s shrinking sea-ice cover. Science 315,
1533–1536.
PubMed Abstract | Publisher Full Text
Stroeve
J.,
Holland
M.M.,
Meier
W.,
Scambos
T.
&
Serreze
M.
2007. Arctic sea ice decline: faster than forecast. Geophysical Research Letters
34, L09501, doi: 10.1029/2007GL029703.
Publisher Full Text