RESEARCH/REVIEW ARTICLE
Trochammina as opportunist foraminifera in the Lower Jurassic from north Siberia
Matías Reolid,1 Boris L. Nikitenko2
& Larissa Glinskikh2
1 Departamento de Geología, Universidad de Jaén, Campus Las Lagunillas sn, ES-23009 Jaén, Spain
2 Institute of Petroleum Geology and Geophysics, Siberian Branch of Russian Academy of Sciences, Ac. Koptyg av. 3 Novosibirsk 90, RU-630090, Russia
Abstract
The ecostratigraphic analysis of foraminiferal assemblages from Upper Pliensbachian to Lower Toarcian (Lower Jurassic) mudstones, siltstones and black shales from northern Siberia allows for a better understanding of the response to the benthic biotic crisis related to the Toarcian Oceanic Anoxic Event in a high latitude context. The assemblages were dominated by agglutinated taxa with extremely low diversity values and dominance of Trochammina. These features suggest that the foraminiferal assemblages were adapted to restricted conditions, where the main limiting factors were salinity and oxygen degree. The opportunist behaviour of Trochammina enabled this genus to survive and adapt to unfavourable conditions. Trochammina proliferated in relation to the sea-level fall and probable changes in salinity in the Arctic palaeobasin during the Margaritatus Chron and at the beginning of the Viligaensis Chron (Late Pliensbachian). Another Trochammina proliferation is associated with the initial development of the restricted oxygen conditions related to the Toarcian Oceanic Anoxic Event.
Keywords
r-strategists; colonization; anoxic event; ecostratigraphy; black shale.
Correspondence
Matías Reolid, Departamento de Geología, Universidad de Jaén, Campus Las Lagunillas sn, ES-23009 Jaén, Spain.
E-mail: mreolid@ujaen.es
(Published: 2 July 2014)
Polar Research 2014. © 2014 M. Reolid et al. This is an Open Access article distributed under the terms of the Creative Commons Attribution-Noncommercial 3.0 Unported License (http://creativecommons.org/licenses/by-nc/3.0/), permitting all non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Citation: Polar Research 2014, 33, 21653, http://dx.doi.org/10.3402/polar.v33.21653
Benthic foraminifera are direct bioindicators of physico-chemical parameters at the sea bottom and indirectly of the water column features (Loubere 1996). Fluctuations in oceanic productivity may enhance the accumulation of organic matter at the sea bottom and affect oxygen demand, exercising a direct influence on the features of the benthic foraminiferal assemblage (diversity, shell composition, morphotypes, etc.). Thus, fluctuations in salinity, nutrient availability and oxygenation rate are controlling parameters for the composition and relative abundance of foraminiferal assemblages (e.g., Sjoerdsma & Van der Zwaan 1992; Jorissen et al. 1995; Van der Zwaan et al. 1999). The depth in the sediment where the foraminifera live is predominantly determined by oxygen and nutrient availability (e.g., Tyszka 1994; Jorissen et al. 1995; Van der Zwaan et al. 1999; Ernst & Van der Zwaan 2004; Reolid, Nagy et al. 2008; Reolid, Rodríguez-Tovar et al. 2008). The epifaunal microhabitat is advantageous in environments with nutrient and/or oxygen limitations, whereas infaunal taxa proliferate if nutrients and oxygen are available. In addition, the opportunist/specialist behaviour of foraminifera and the diversity of the assemblages are related to nutrient input; a nutrient increase favours the proliferation of opportunist taxa (r-type strategy) and produces the diminution of foraminiferal diversity (Sjoerdsma & Van der Zwaan 1992). For this reason, foraminifera provide insights as to the controlling factors behind Jurassic and Cretaceous oceanic anoxic events and the response of microfaunal assemblages before and after an event (e.g., Bartolini et al. 1992; Hylton & Hart 2000; Coccioni & Luciani 2004; Gebhardt et al. 2004; Friedrich et al. 2009; Mailliot et al. 2009; Nikitenko 2009; Soua et al. 2011; Reolid, Rodríguez-Tovar & Nagy 2012; Reolid, Sebane et al. 2012). The r-strategist genera have an important role in such biotic crises, as subsequent survivors or colonizers (e.g., Rey et al. 1994; Tyszka 1994; Reolid et al. 2010; Reolid, Rodríguez-Tovar, Marok et al. 2012; Reolid, Sebane et al. 2012). In nutrient-rich environments (eutrophic waters), r-strategists (opportunists) proliferate, the rapid increase of their population densities characterized by faster reproduction and generally smaller size (e.g., MacArthur & Wilson 1967; Valentine 1973; Hallock 1985). In low-nutrient environments (oligotrophic waters), K-strategists (specialists) dominate, in turn characterized by long individual life spans, a low reproductive rate and larger shell size (e.g., MacArthur & Wilson 1967; Valentine 1973; Hallock et al. 1991). Generally, the trophic conditions are also related to oxygen availability, with restricted oxygenation in eutrophic waters and abundant oxygenation in oligotrophic waters.
In the case of the Early Toarcian Oceanic Anoxic Event (T-OAE), a foremost environmental change during the Mesozoic resulted in a mass extinction of benthic groups in marine ecosystems (Wignall et al. 2005). In general, the T-OAE is characterized by a record of organic-rich sediments associated with a negative excursion in δ13C (e.g., Jenkyns & Clayton 1997; Röhl et al. 2001; Hesselbo et al. 2007; Hermoso et al. 2009; Suan et al. 2011), in a context of exceptionally warm conditions (e.g., McArthur et al. 2000; Svensen et al. 2007; Gómez & Goy 2011; Suan et al. 2011) and sea-level rise (e.g., Haq et al. 1987; Hallam 2001; Nikitenko 2008, 2009). For the T-OAE, the genera of calcitic and aragonitic foraminifera identified as opportunists (r-strategists) in the Tethys Realm would include Lenticulina, Eoguttulina and Reinholdella (Nocchi & Bartolini 1994; Boutakiout & Elmi 1996; Reolid, Sebane et al. 2012). However, ecostratigraphic analyses of T-OAE-related foraminiferal assemblages in the Boreal Domain, where agglutinated forms dominate, are scarce (Nagy & Johansen 1991; Nagy 1992; Nikitenko & Mickey 2004; Nikitenko 2008). The present contribution is an ecostratigraphic analysis of the foraminiferal assemblages during the T-OAE, with identification of the r-strategist genera at high latitudes.
Geological setting
The Kelimyar River section is located in north-eastern Siberia (Fig. 1), close to the Laptev Sea in the Arctic Ocean. This section exposes an Upper Pliensbachian to Lower Toarcian marine succession made up of sandy siltstones, siltstones and shale clay. These sediments were deposited in a continental shelf environment (Nikitenko 20082009), in the upper sublittoral zone during the Latest Pliensbachian and the middle sublittoral zone during the Early Toarcian (Fig. 2). According to plate tectonic reconstructions, the Kelimyar River district was located several kilometres south-west from the Early Jurassic magnetic North Pole, which was in the delta area of the Lena River (Golonka & Scotese 1995; Ford & Golonka 2003; Golonka et al. 2003; Golonka 2007, 2011; Scotese 2011; Torsvik et al. 2012; Fig. 2). These data agree with biogeographic and palaeogeographic reconstructions (Nikitenko 2008). The 14.6 m-thick interval studied in this work takes in the Margaritatus Zone to the top of Falciferum Zone. The Upper Pliensbachian corresponds to the Kyra Formation and the Toarcian part of the section corresponds to the Kelimyar Formation (Fig. 1e). A condensed Antiquum Zone of a few centimetres characterizes the succession. The Falciferum Zone is represented by the Kurung Member, with finely laminated black shales, high values of total organic carbon (TOC, 6 wt.%) and a negative carbon isotopic excursion (Suan et al. 2011).
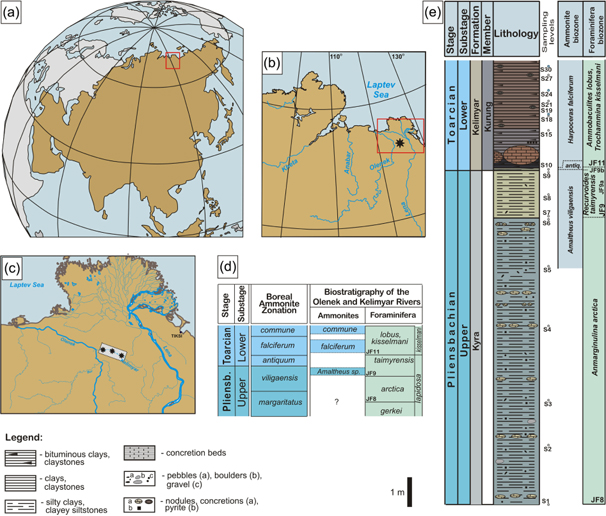
Fig. 1
(a, b, c) Geographic location of Kelimyar River Kelimyar River section (e) with location of the sampling levels and (d) and foraminiferal and ammonite biostratigraphy of the Olenek–Kelimyar rivers area (based on Nikitenko 2009) compared to equivalent Boreal and north-west European ammonite zones. Colours in the lithological column correspond to field outcrop appearance.
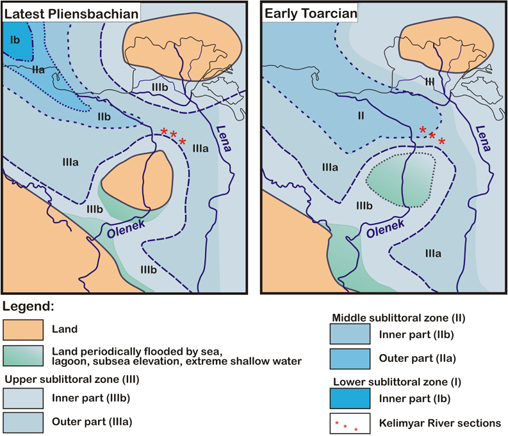
Fig. 2
Palaeogeography of the Anabar–Lena Sea in Late Pliensbachian and Early Toarcian.
Material and methods
Analysis of foraminiferal assemblages was undertaken from 17 sampling levels (Fig. 1e). Each sample (200 g) was soaked in tap water for a few days and later disintegrated in boiled water and rinsed through a 56-µm sieve mesh. The residue of each sample was totally retrieved with a very variable amount of picked individuals per sample (Table 1). There were very low numbers of specimens in some samples (S10, S24, S27 and S30; see Table 1). A total of more than 2900 foraminifera were analysed. Foraminiferal analysis was focused mainly on proportions (%) and abundance of foraminifera (specimen/100 g) with different life-styles (epifaunal, shallow infaunal and potentially deep infaunal) based on the morphogroup interpretations of Nikitenko et al. (2013). The characterization of benthic foraminiferal morphogroups in view of palaeoenvironmental assessments has sparked great interest over the last two decades (e.g., Nagy 1992; Tyszka 1994; Reolid, Rodríguez-Tovar et al. 2008; Nagy et al. 2009; Reolid et al. 2010; Murray et al. 2011; Reolid, Sebane et al. 2012; Setoyama et al. 2013). Studies of modern and ancient foraminiferal assemblages demonstrate that the morphology of the foraminiferal shell (general shell morphology, aperture position, mode of coiling and number of chambers) can be directly related to different life-styles and feeding strategies (e.g., Jones & Charnock 1985; Corliss & Chen 1988; Corliss 1991; Nagy 1992; Tyszka 1994; Setoyama et al. 2013). In addition, α-diversity based on genera (Fisher et al. 1943) was included in the analysis of foraminiferal assemblages. Diversity analysis was based on genera because the taxonomy of some species varies considerably in different publications, whereas the nomenclature of genera is more stable. The samples are housed in the Micropalaeontology Laboratory of the Institute of Petroleum Geology and Geophysics, of the Siberian Branch of the Russian Academy of Sciences (Nikitenko, personal collection).
Table 1 Original count of foraminifera retrieved from 200 g of dried samples from the Kelimyar River section.
|
Stage |
|
Pliensbachian |
Toarcian |
| Sample |
S1 |
S2 |
S3 |
S4 |
S5 |
S6 |
S7 |
S8 |
S9 |
S10 |
S15 |
S18 |
S19 |
S21 |
S24 |
S27 |
S30 |
| Ammobaculites
|
1 |
0 |
0 |
0 |
0 |
1 |
2 |
2 |
1 |
1 |
2 |
0 |
0 |
0 |
0 |
0 |
0 |
| Ammodiscus
|
10 |
4 |
13 |
5 |
60 |
34 |
55 |
15 |
32 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
2 |
| Anmarginulina
|
1 |
2 |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
| Astacolus
|
0 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
| Bulbobaculites
|
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
2 |
12 |
7 |
10 |
4 |
0 |
0 |
| Conorboides
|
27 |
6 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
| Dentalina
|
9 |
6 |
2 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
6 |
0 |
0 |
0 |
0 |
| Eoguttulina
|
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
10 |
0 |
0 |
0 |
0 |
| Evolutinella
|
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
10 |
25 |
20 |
0 |
11 |
0 |
| Globulina
|
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
15 |
0 |
0 |
0 |
0 |
| Glomospira
|
3 |
0 |
0 |
7 |
12 |
1 |
10 |
4 |
17 |
10 |
2 |
2 |
0 |
0 |
0 |
1 |
0 |
| Glomospirella
|
10 |
0 |
5 |
7 |
0 |
0 |
0 |
0 |
2 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
| Hyperammina
|
10 |
21 |
50 |
9 |
70 |
36 |
50 |
20 |
15 |
1 |
1 |
12 |
1 |
70 |
2 |
0 |
0 |
| Ichthyolaria
|
1 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
| Jaculella
|
0 |
0 |
0 |
2 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
| Kutsevella
|
0 |
0 |
2 |
0 |
6 |
6 |
10 |
7 |
3 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
| Lagenammina
|
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
6 |
4 |
0 |
0 |
0 |
| Lenticulina
|
2 |
2 |
2 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
2 |
0 |
0 |
0 |
0 |
| Marginulina
|
2 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
| Nodosaria
|
1 |
1 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
| Palmula
|
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
1 |
0 |
0 |
0 |
0 |
| Pyrulinoides
|
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
| Recurvoides
|
0 |
0 |
0 |
5 |
0 |
0 |
80 |
70 |
80 |
1 |
3 |
0 |
3 |
5 |
0 |
0 |
0 |
| Reophax
|
1 |
0 |
0 |
1 |
0 |
3 |
7 |
10 |
0 |
0 |
0 |
7 |
6 |
12 |
0 |
3 |
0 |
| Saccammina
|
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
4 |
0 |
8 |
0 |
0 |
| Spiroplectammina
|
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
4 |
5 |
0 |
5 |
0 |
0 |
0 |
| Tolypammina
|
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
29 |
0 |
0 |
0 |
| Trochammina
|
32 |
115 |
60 |
45 |
180 |
124 |
20 |
40 |
30 |
10 |
85 |
500 |
150 |
120 |
30 |
35 |
10 |
| Verneuilinoides
|
2 |
1 |
0 |
0 |
4 |
0 |
0 |
0 |
0 |
0 |
2 |
0 |
1 |
0 |
0 |
0 |
0 |
Foraminiferal assemblages: results
The studied foraminiferal assemblages consist of benthic forms belonging to the suborders Textulariina, Lagenina and Robertinina. Agglutinated taxa are dominant (18 genera) and taxa with calcareous-perforated shell forms are secondary (11 calcitic hyaline and 1 aragonitic hyaline). Among the agglutinated forms, Trochammina lapidosa is dominant in the Upper Pliensbachian and Trochammina kisselmani is dominant in the Lower Toarcian samples (Fig. 3). These assemblages often comprise well-preserved tests and carapaces without any trace of grading or transportation. Calcareous foraminifera in the latest Pliensbachian and earliest Toarcian were rare. This is not a taphonomic effect, however, because they did not dissolve in the water column of the Anabar–Lena palaeosea. The lysocline level is located at a depth of 1–4 km in modern cold seas, while in Jurassic warm seas, this level should be at a greater depth (e.g., Butler 1982; Thurman & Trujillo 2004). The study area (Kelimyar River district) was located in a mid-shelf environment, in the photic zone of the Anabar–Lena palaeosea, and therefore the depth of this area does not exceed 80–100 m (Nikitenko 2008). Foraminifera with aragonite walls should begin to dissolve at a much shallower depth, but they are present in the section (Pliensbachian). In the Toarcian samples common ostracods are recorded, having well-preserved sculptured carapaces with thin spikes and no evidence of dissolution (Nikitenko 2008, 2009). In addition, the shells of ammonites, belemnites and bivalves do not bear traces of dissolution either. For these reasons, the composition of foraminiferal assemblages is not severely affected by taphonomic processes.
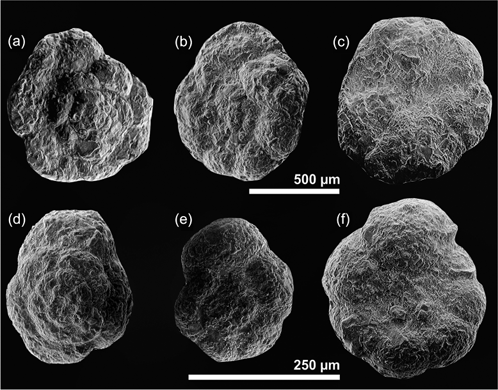
Fig. 3
Scanning electron images of (a, b, c) Trochammina lapidosa, Upper Pliensbachian, microsphaeric form, (a) dorsal view, (b, c) megalosphaeric form, (b) dorsal view, (c) ventral view and (d, e, f) Trochammina kisselmani, Lower Toarcian, (d, f) microsphaeric form, (d) dorsal view, (f) ventral view, megalosphaeric form, (e) dorsal view.
Trochammina, the dominant foraminifera in the studied section, is a globular and plano-convex low trochospiral foraminifera interpreted as epifaunal, with a feeding strategy of detritivore or bacterivore and probably active herbivore, including phytodetritus corresponding to the morphogroup D1 of Reolid et al. (2010) and morphogroup D of Nikitenko et al. (2013).
The base of the Upper Pliensbachian is characterized by the dominance of epifauna (Fig. 4) represented mainly by Trochammina and, secondarily, Conorboides, Ammodiscus, Hyperammina, Glomospirella and Lagenina (sample 1). Aragonitic and calcitic foraminifera are present (24 and 15%, respectively) but with decreasing trends from sample 1 to 3, and eventually they disappear (sample 4). The abundance of foraminifera is relatively high (ca. 60 specimens/100 g) at the base of the section (sample 1). Most of the Kyra Member (Upper Pliensbachian) is characterized by dominance of Trochammina (>50% of the assemblage; Fig. 4), increasing values of Ammodiscus and Hyperammina, and reduced numbers of Glomospira, Reophax, Kutsevella, and Recurvoides. However, the last 1.5 m of the Upper Pliensbachian shows an abrupt decrease of Trochammina mainly compensated by increasing proportions of Recurvoides (Fig. 5). Throughout the Kyra Member infaunal forms decrease (Fig. 4). The analysis of the genus Trochammina from the Upper Pliensbachian of Kelimyar section and other outcrops of Siberia evidences that the species determined earlier as Trochammina lapidosa, Trochammina ex gr. inflata, Trochammina inflataeformis and Trochammina sablei, should in fact be attributed to one species, T. lapidosa (Nikitenko 2009). At the base of the studied section the diversity of foraminifera is higher; diversity then decreases with the increasing abundance of foraminifera, mainly corresponding to Trochammina, except for an abrupt decrease in the topmost Pliensbachian. If we consider the abundance of Trochammina in light of the abundance of the rest of foraminifera (Figs. 6, 7), it is clear that only Trochammina dramatically decrease while the other foraminifera maintain the values of abundance or increase (mainly Recurvoides and Ammodiscus). Considering percentages, in the topmost Pliensbachian the proportions of Recurvoides taimyrensis abruptly increase (44%), whereas proportions of the genera Trochammina lapidosa decrease (<24%; Fig. 5). In this uppermost part of the Pliensbachian, infaunal forms experiment a brief increase (Fig. 4). The α-diversity index and the number of genera drop during the Late Pliensbachian (α-diversity index from 5 to values usually<2; Fig. 7).
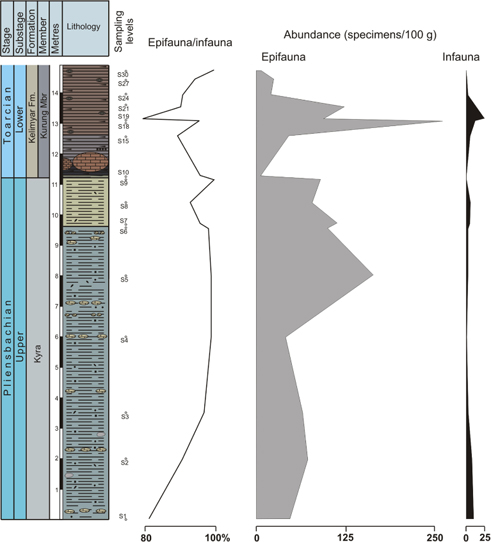
Fig. 4
Ecostratigraphic distribution of epifauna/infauna ratio (expressed as%) and abundance (specimen per 100 g) of epifaunal and infaunal foraminifera in the Kelimyar River section.
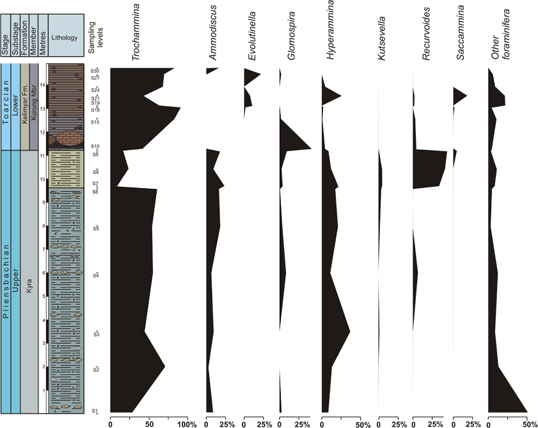
Fig. 5
Ecostratigraphic distribution of proportions of selected epifaunal foraminifera including Trochammina in the Kelimyar River section.
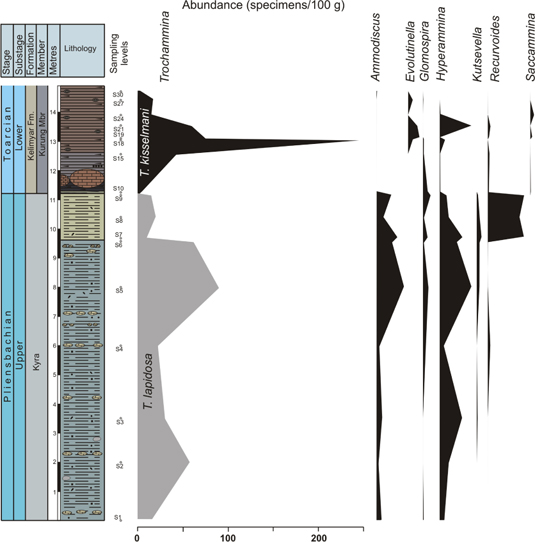
Fig. 6
Ecostratigraphic distribution of abundance (specimen per 100 g) of selected epifaunal foraminifera including Trochammina in the Kelimyar River section.
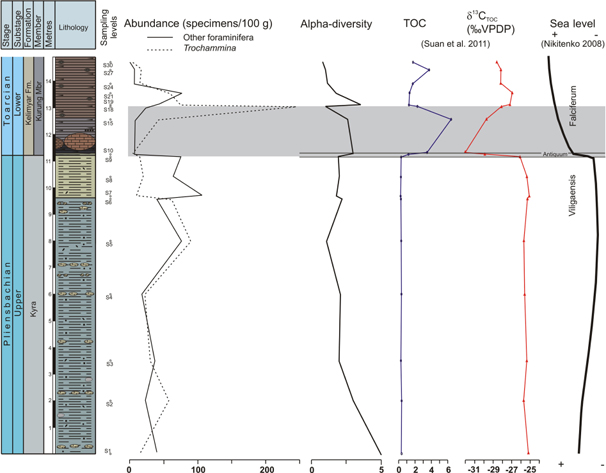
Fig. 7
Abundance of Trochammina vs. rest of foraminifera compared with α-diversity index, as well as the total organic carbon (TOC) and δ13C from organic matter of Suan et al. (2011), and sea-level fluctuations according to Nikitenko (2008).
The lowermost part of the Lower Toarcian in this section (more precisely, the Antiquum–Falciferum biozone boundary) is characterized by an abrupt increase of TOC (maximum 6 wt.%; Fig. 7) and a negative carbon isotopic excursion of 6‰ (δ13CTOC of −32‰, Suan et al. 2011; Fig. 7). These values persist 1.8 m over the Pliensbachian–Toarcian boundary. The foraminifera present an abrupt decline in abundance here (Figs. 6, 7), with the transition to black shales (12 specimens/100 g), and the disappearance of Ammodiscus and Kutsevella. At this boundary the assemblage is dominated by Trochammina (42%) and Glomospira (42%); meanwhile, Recurvoides taimyrensis dramatically decreases (Fig. 5). In the Lower Toarcian Trochammina is represented by T. kisselmani. During the stratigraphic interval (1.8 m) with the highest TOC values and the negative excursion of δ13CTOC, the proportion of Trochammina increases rapidly (Fig. 5). Taking into account the abundance of foraminifera, only Trochammina increases in the black shales (from sample 10 to 15; Fig. 6). Just at the top of this stratigraphic interval (1.8 m, sample 18), an abrupt recovery is recorded (274 specimens/100 g) led by Trochammina (91% of the assemblage) and some infaunal forms (Figs. 4–6), though characterized by low α-diversity values. The recovery of other foraminifera (sample 21) is delayed with respect to the acme of Trochammina (sample 18, Figs. 5, 6). The diameters of both megalosphaeric and microsphaeric generations of T. kisselmani are about two to three times shorter than T. lapidosa (Table 2). In general, after the biotic crisis at the beginning of the Early Toarcian, the size of foraminifera (all taxa) in the assemblages is two to three times lower than in the Late Pliensbachian.
Table 2 Data on tests of Trochammina lapidosa and Trochammina kisselmani from 50 individuals.
| Generation |
Diameter of prolocus (mm) |
Diameter (mm) |
Height of test (mm) |
No. of convolutions |
| Trochammina lapidosa Gerke et Sossipatrova, 1961 |
| Megalosphaeric |
0.060–0.084 |
0.42–0.78 |
0.14–0.27 |
1.2–2.0 |
| Microsphaeric |
0.014–0.028 |
0.64–0.95 |
0.27–0.36 |
2.5–4.0 |
| Trochammina kisselmani Sapjanik et Sokolov, 1991 |
| Megalosphaeric |
0.020–0.070 |
0.25–0.44 |
0.098–0.150 |
1.0–3.0 |
| Microsphaeric |
0.012–0.020 |
0.21–0.31 |
0.160–0.210 |
3.0–4.0 |
Trochammina and palaeoenvironmental fluctuations
Benthic habitat fluctuations in salinity, nutrient availability and oxygenation rate are limiting palaeoenvironmental parameters, exerting considerable control upon the composition and relative abundance of foraminiferal assemblages from the studied section.
The Upper Pliensbachian is represented by a foraminiferal assemblage dominated by epifauna, with a high proportion of Trochammina lapidosa, yet a progressive decline in the diversity and number of genera. This suggests an unfavourable microhabitat for infaunal forms (e.g., Ammobaculites, Dentalina, Lenticulina, Nodosaria, Pyrulinoides, Reophax, Verneuilinoides), probably involving salinity fluctuations. Trochammina has been proposed as an opportunist epifaunal taxon tolerating salinity fluctuations (Nagy & Berge 2008; Nagy et al. 2010). Low diversity assemblages of agglutinated foraminifera dominated by Trochammina appear to have been widespread in Late Triassic to Middle Jurassic restricted environments along the north-western and western European margins (Golebiowski 1990; Kuerschner et al. 2007; Clémence et al. 2010; Nagy et al. 2010). The decreasing proportions of aragonitic and calcitic hyaline tests (Anmarginulina, Astacolus, Conorboides, Dentalina, Ichthyolaria, Lenticulina, Marginulina, and Pyrulinoides) confirm the progressive destabilization of the benthic environment. This interpretation is congruent with the context of relative sea-level fall in the Arctic palaeobasin during the Margaritatus Chron and the early Viligaensis Chron (Nikitenko & Mickey 2004). In conjunction with this eustatic fall, abrupt climatic cooling due to the presence of glendonites (calcite pseudomorph after ikaite) has been evoked for north-eastern Siberia (Kaplan 1976; Suan et al. 2011). A sea-level fall has likewise been interpreted for the Pliensbachian–Toarcian boundary in other regions, as well as a drastic cooling event during the last ammonite zone of the Pliensbachian (e.g., Price 1999; Rosales et al. 2004; Van de Schootbrugge et al. 2005; Suan et al. 2010; Dera et al. 2011; Korte & Hesselbo 2011).
The topmost 1.5 m of the Pliensbachian records an improvement of environmental features evidenced by an enhanced abundance of foraminifera excepting opportunist Trochammina. The favourable conditions are also compatible with the brief increase of infaunal forms (Fig. 4) such as Reophax and Ammobaculites (Table 1).
The beginning of the Lower Toarcian (exactly at the Antiquum-Falciferum zone boundary) is correlated with the T-OAE (increase of TOC and negative carbon isotopic excursion). This event is marked in the Kelimyar River section by an abrupt reduction in the abundance of foraminifera (Figs. 6, 7), indicating hypoxic conditions at sea bottom. Totally anoxic conditions apparently did not develop, in view of the record of benthic foraminifera (no benthic barren interval). According to Nikitenko & Mickey (2004), monospecific associations of thin-shelled bivalves and thin-shelled ostracods recorded in this interval would confirm the development of oxygen- restricted biofacies. Among the foraminifera, only r-strategist Trochammina kisselmani shows a rapid increase after the debut of the black shales, congruent with the association of this genus with a high TOC and poorly oxygenated sediment water interface. Trochammina has been proposed as an opportunist epifaunal taxon that tolerates low oxygenation (Bąk 2000; Jenkins 2000; Reolid & Nagy 2008; Reolid et al. 2010), and it has also been registered along with high TOC values after anoxic or suboxic conditions during the Middle Jurassic to Early Cretaceous (e.g., Nyong & Ramanathan 1985; Koutsoukos & Hart 1990; Friedrich et al. 2003; Reolid & Nagy 2008; Reolid, Nagy et al. 2008; Reolid, Rodríguez-Tovar & Nagy 2012). In recent environments Trochammina has been seen to increase rapidly in the surficial sediment to consume labile organic matter (Koho et al. 2008), and to survive in environments under hypoxic and eutrophic conditions (Tsujimoto et al. 2006) as well as in marshes with extreme chemical pollution and low pH (McGann & Sloan 1999).
The diameter of foraminifera decreases at the beginning of the Early Toarcian, especially in the case of Trochammina. This strategy, enabling them to prosper in more or less confined environments, is known as the Lilliput Effect (Twitchett 2007; Morten & Twitchett 2009; Song et al. 2011) and represents a decrease within the surviving species. This has been described as an adaptative strategy in miliolids and lagenids of the Toarcian from the Middle Atlas of Morocco (Reolid et al. 2013).
Without tough competition, Trochammina rapidly reproduced and augmented its population (Figs. 6 and 7). This record is similar to that described in the uppermost Triassic of the Austrian Alps by Clémence et al. (2010), where an event characterized by a negative carbon isotopic excursion, an increase in TOC and a barren foraminiferal interval is followed by an abundance peak of Trochammina. These authors use the term “disaster epifaunal agglutinated foraminifera” to describe such Trochammina peaks. In this sense, Trochammina kisselmani was an effective colonizer of the bottom after hypoxic conditions in the Kelimyar River section, indicating stressful conditions just over the negative carbon isotopic excursion and maximum values of TOC. The advance in the Kurung Member shows a new decrease in abundance and diversity. According to Nikitenko et al. (2013), this new retrocession in the foraminiferal assemblages also affecting Trochammina may be related to the persistence of adverse conditions upon foraminiferal assemblages only partially recovered after the debut of the T-OAE.
The stressing environmental conditions related to changes in oxygenation in the sea bottom were driven by climatic changes in the Pliensbachian–Toarcian boundary. According to Nikitenko & Mickey (2004), during the Early Toarcian a climatic warming took place in the Arctic palaeobasins, related to a major eustatic rise. A sea-level rise accompanied by an abrupt warming event has been interpreted for the Early Toarcian in other regions as well (e.g., McArthur et al. 2000; Svensen et al. 2007; Gómez et al. 2008; Suan et al. 2010; Dera et al. 2011; Gómez & Goy 2011; Korte & Hesselbo 2011). The record of ice-rafted boulders and glendonites supports the—at least—intermittent formation of polar sea ice during the Late Pliensbachian in this area (Suan et al. 2011). The subsequent warming during the earliest Toarcian—increasing 6–10°C in some areas (Bailey et al. 2003; Gómez et al. 2008; Suan et al. 2010)—indicates a probable glacially induced sea-level rise due a massive melting of continental ice (Suan et al. 2011). Hallam (1997) interpreted an abrupt sea-level rise about 30–90 m between the uppermost Pliensbachian and the negative δ13C excursion of the Lower Toarcian in European sections. This situation evidently changed the sea-bottom features and produced stressing conditions for benthic foraminifera.
Another essential point collaborating in adverse conditions is that such high palaeolatitudes are always strongly seasonal. This was most likely a main driving force for true opportunists (see the case of Trochammina hadai, Kitazato & Matsushita 1996). Tyszka (2009, 2010) proposes the term seasonal opportunists when seasonality is a dominating environmental factor though not usually recognized in average fossil assemblages.
Conclusions
The samples of benthic foraminifera analysed correspond to mudstones and siltstones deposited in a middle to upper sublittoral zone. The section is located in northern Siberia and includes the Upper Pliensbachian and the Lower Toarcian (Lower Jurassic). Salient features of the foraminiferal successions are: the assemblages are dominated by agglutinated taxa; the α-diversity values are extremely low, and the dominant genus is Trochammina.
These characteristics suggest that the foraminiferal assemblages were adapted to restricted conditions, where the main limiting factors were salinity and oxygen degree. However, due the high palaeolatitude, seasonality could be a dominant environmental factor altering temperature, nutrients, light, runoff, etc.; the opportunists simply reacted to seasonal food fluxes, explaining why Trochammina dominates in nearly all samples.
Episodes featuring a higher proliferation of Trochammina may be related to: (a) sea-level fall and consequent changes in salinity in the Arctic Palaeobasin during the Margaritatus Chron and the beginning of the Viligaensis Chron (Late Pliensbachian), and (b) restricted oxygen biofacies (hypoxic conditions) related to the T-OAE.
The opportunist behaviour of Trochammina, making possible its survival and adaptation to unfavourable conditions, led this genus to be the main colonizer of the sea bottom after the biotic crisis related to the T-OAE in this section.
Acknowledgements
The research activity of MR was supported by projects RYC-2009-04316 (Ramón y Cajal Programme), P11-RNM-7408 (Junta de Andalucía) and UJA2011/12/17 (Universidad de Jaén-Caja Rural de Jaén). For their research support, BLN and LG gratefully acknowledge the Arctica and Biosphere scientific programmes of the Russian Academy of Sciences. The authors thank two anonymous reviewers for their valuable comments serving to improve the manuscript. We thank Jean Sanders for reviewing the grammar.
References
Bailey
T.R.,
Resenthal
Y.,
McArthur
J.M.,
van de Schootbrugge
B.
&
Thirlwall
M.F. 2003.
Paleoceanographic changes of the Late Pliensbachian–Early Toarcian interval: a possible link to the genesis of an oceanic anoxic event. Earth and Planetary Science Letters 212, 307–320.
Publisher Full Text
Bak
K. 2000.
Biostratigraphy of deep-water agglutinated foraminifera in Scaglia Rossa-type deposits of the Pienniny Klippen Belt, Carpathians, Poland. Grzybowski Foundation Special Publication 7, 15–41.
Bartolini
A.,
Nocchi
M.,
Baldanza
A.
&
Parisi
G. 1992. Benthic life during the Early Toarcian Anoxic Event in the south western Tethyan Umbria-Marche Basin, central Italy. In
Y.
Takayanagi
&
T.
Saito (eds.): Studies in benthic foraminifera. Proceedings of the Fourth International Symposium on Benthic Foraminifera, Sendai, Japan, 1990. Pp. 323–338.
Sendai: Tokai University Press.
Boutakiout
M.
&
Elmi
S. 1996.
Tectonic and eustatic controls during the Lower and Middle Jurassic of the South Rif Ridge (Morocco) and their importance for the foraminifera-communities. Georesearch Forum 1–2, 237–247.
Butler
J.N. 1982. Carbon dioxide equilibria and their applications. Reading, MA: Addison-Wesley.
Clémence
M.E.,
Gardin
S.,
Bartolini
A.,
Paris
G.,
Beaumont
V.
&
Guex
J. 2010.
Bentho-planktonic evidence from the Austrian Alps for a decline in sea-surface carbonate production at the end of the Triassic. Swiss Journal of Geosciences 103, 293–315.
Publisher Full Text
Coccioni
R.
&
Luciani
V. 2004.
Planktonic foraminifera and environmental changes across the Bonarelli Event (OAE2, Latest Cenomanian) in its type area: a high-resolution study from the Tethyan reference Bottaccione section (Gubbio, Central Italy). Journal of Foraminiferal Research 34, 109–129.
Publisher Full Text
Corliss
B.H. 1991.
Morphology and microhabitat preferences of benthic foraminifera from the northwest Atlantic Ocean. Marine Micropaleontology 17, 195–236.
Publisher Full Text
Corliss
B.H.
&
Chen
C. 1988.
Morphotype patterns of Norwegian deep sea benthic foraminifera and ecological implications. Geology 16, 716–719.
Publisher Full Text
Dera
G.,
Brigaud
B.,
Monna
F.,
Laffont
R.,
Pucéat
E.,
Deconinck
J.F.,
Pellenard
P.,
Joachimski
M.M.
&
Durlet
C. 2011.
Climatic ups and downs in disturbed Jurassic world. Geology 39, 215–218.
Publisher Full Text
Ernst
S.
&
Van der Zwaan
B. 2004.
Effects of experimentally induced raised levels of organic flux and oxygen depletion on a continental slope benthic foraminiferal community. Deep-Sea Research Part I 51, 1709–1739.
Publisher Full Text
Fisher
R.A.,
Corbet
A.S.
&
Williams
C.B. 1943.
The relations between the number of species and the number of individuals in a random sample of an animal population. Journal of Animal Ecology 12, 42–58.
Publisher Full Text
Ford
D.
&
Golonka
J. 2003.
Phanerozoic paleogeography, paleoenvironment and lithofacies maps of the circum-Atlantic margins. Marine and Petroleum Geology 20, 249–285.
Publisher Full Text
Friedrich
O.,
Erbacher
J.,
Wilson
P.A.,
Moriya
K.
&
Mutterlose
J. 2009.
Paleoenvironmental changes across the Mid Cenomanian Event in the tropical Atlantic Ocean (Demerara Rise ODP Leg 207) inferred from benthic foraminiferal assemblages. Marine Micropaleontology 71, 28–40.
Publisher Full Text
Friedrich
O.,
Reichelt
K.,
Herrle
J.O.,
Lehmann
J.,
Pross
J.
&
Hemleben
C. 2003.
Formation of the Late Aptian Niveau Fallot black shales in the Vocontian Basin (SE France): evidence from foraminifera, palynomorphs, and stable isotopes. Marine Micropaleontology 49, 65–85.
Publisher Full Text
Gebhardt
H.,
Kuhnt
W.
&
Holbourn
A. 2004.
Foraminiferal response to sea level change, organic flux and oxygen deficiency in the Cenomanian of the Tarfaya Basin, southern Morocco. Marine Micropaleontology 53, 133–157.
Publisher Full Text
Golebiowski
R. 1990.
Facial and faunistic changes from Triassic to Jurassic in the northern Calcareous Alps. Cahiers de l'Université de Lyon 3, 175–184.
Golonka
J. 2007.
Late Triassic and Early Jurassic paleogeography of the world. Palaeogeography, Palaeoclimatology, Palaeoecology 244, 297–307.
Publisher Full Text
Golonka
J. 2011.
Phanerozoic palaeoenvironment and palaeolithofacies maps of the Arctic region.
In A.M. Spencer & (eds.): Arctic petroleum geology. Pp. 79–129. London: Geological Society.
Golonka
J.,
Bocharova
N.Y.,
Ford
D.,
Edrich
M.E.,
Bednarczyk
J.
&
Wildharber
J. 2003.
Paleogeographic reconstructions and basins development of the Arctic. Marine and Petroleum Geology 20, 211–248.
Publisher Full Text
Golonka
J.
&
Scotese
C.R. 1995.
Phanerozoic paleogeographic maps of Arctic margins.
In K.V. Simakov & D.K. Thurston (eds.): Proceedings of International Conference on Arctic Margins. Magadan, Russia, September 1994. Pp. 1–16. Magadan: Russian Academy of Sciences, Far Eastern Branch.
Gómez
J.J.
&
Goy
A. 2011.
Warming-driven mass extinction in the Early Toarcian (Early Jurassic) of northern and central Spain. Correlation with other time-equivalent European sections. Palaeogeography, Palaeoclimatology, Palaeoecology 306, 176–195.
Publisher Full Text
Gómez
J.J.,
Goy
A.
&
Canales
M.L. 2008.
Seawater temperature and carbon isotope variations in belemnites linked to mass extinction during the Toarcian (Early Jurassic) in central and northern Spain. Comparison with other European sections. Palaeogeography, Palaeoclimatology, Palaeoecology 258, 28–58.
Publisher Full Text
Hallam
A. 1997.
Estimates of the amount and rate of sea-level change across the Rhaetian-Hettangian and Pliensbachian-Toarcian boundaries (latest Triassic to early Jurassic). Journal of the Geological Society, London 154, 773–779.
Publisher Full Text
Hallam
A. 2001.
A review of the broad pattern of Jurassic sea-level changes and their possible causes in the light of current knowledge. Palaeogeography, Palaeoclimatology, Palaeoecology 167, 23–37.
Publisher Full Text
Hallock
P. 1985.
Why are larger foraminifera large? Paleobiology 11, 195–208.
Hallock
P.,
Premoli Silva
I.
&
Boersma
A. 1991.
Similarities between planktonic and larger foraminiferal evolutionary trends through Paleogene evolutionary trend. Palaeogeography, Palaeoclimatology, Palaeoecology 84, 49–64.
Publisher Full Text
Haq
B.U.,
Hardenbol
J.
&
Vail
P.R. 1987.
Chronology of fluctuating sea level since the Triassic. Science 235, 1156–1167.
Publisher Full Text
Hermoso
M.,
Le Callonnec
L.,
Minoletti
F.,
Renard
M.
&
Hesselbo
S.P. 2009.
Expression of the Early Toarcian negative carbon-isotope excursion in separated carbonate microfractions (Jurassic, Paris Basin). Earth and Planetary Science Letters 277, 194–203.
Publisher Full Text
Hesselbo
S.P.,
Jenkyns
H.C.,
Duarte
L.V.
&
Oliveira
L.C.V. 2007.
Carbon isotope record of the Early Jurassic (Toarcian) Oceanic Anoxic Event from fossil wood and marine carbonate (Lusitanian Basin, Portugal). Earth and Planetary Science Letters 253, 455–470.
Publisher Full Text
Hylton
M.D.
&
Hart
M.B. 2000.
Benthic foraminiferal response to Pliensbachian-Toarcian (Lower Jurassic) sea-level change and oceanic anoxia in NW Europe. Georesearch Forum 6, 455–462.
Jenkins
C.D. 2000.
The ecological significance of foraminifera in the Kimmeridgian of Southern England. Grzybowski Foundation Special Publication 7, 167–178.
Jenkyns
H.C.
&
Clayton
C.K. 1997.
Lower Jurassic epicontinental carbonates and mudstones from England and Wales: chemostratigraphic signals and the early Toarcian anoxic event. Sedimentology 44, 687–706.
Publisher Full Text
Jones
R.W.
&
Charnock
M.A. 1985.
“Morphogroups” of agglutinating foraminifera. Their life position and feeding habits and potential applicability in (paleo)ecological studies. Revue de Paléobiologie 4, 311–320.
Jorissen
F.J.,
De Stigter
H.C.
&
Widmark
J.G.V. 1995.
A conceptual model explaining benthic foraminiferal habitats. Marine Micropaleontology 26, 3–15.
Publisher Full Text
Kaplan
M.E. 1976. Litologija morskih mezozojskih otlo(enij severa vostocnoj Sibiri. (Lithology of marine Mesozoic deposits in the northern part of Eastern Siberia.) Leningrad: Nedra Leningrad.
Kitazato
H.
&
Matsushita
S. 1996.
Laboratory observations of sexual and asexual reproduction of Trochammina hadai Uchio. Transactions and Proceedings of the Palaeontological Society of Japan, New Series 182, 454–466.
Koho
K.A.,
Langezaal
A.M.,
van Lith
Y.A.,
Duijnstee
I.A.P.
&
van der Zwaan
G.J. 2008.
The influence of a simulated diatom bloom on deep-sea benthic foraminifera and the activity of bacteria: a mesocosm study. Deep-Sea Research Part I 55, 696–719.
Publisher Full Text
Korte
C.
&
Hesselbo
S.P.
2011. Shallow marine carbon and oxygen isotope and elemental records indicate icehouse–greenhouse cycles during the Early Jurassic. Paleoceanography
26, PA4219, doi: 10.1029/2011PA002160.
Publisher Full Text
Koutsoukos
E.A.M.
&
Hart
M.B. 1990.
Cretaceous foraminiferal morphogroup distribution patterns, paleocommunities and trophic structures—a case-study from the Serpige Basin, Brazil. Transactions of the Royal Society of Edinburgh-Earth Sciences 81, 221–246.
Publisher Full Text
Kuerschner
W.M.,
Bonis
N.R.
&
Krystyn
L. 2007.
Carbon isotope stratigraphy and palynostratigraphy of the Triassic-Jurassic transition in the Tiefengraben section—Northern Calcareous Alps (Austria). Palaeogeography, Palaeoclimatology, Palaeoecology 244, 257–280.
Publisher Full Text
Loubere
P. 1996.
The surface ocean productivity and bottom water oxygen signals in deep water benthic foraminiferal assemblages. Marine Micropaleontology 28, 247–261.
Publisher Full Text
MacArthur
R.H.
&
Wilson
E.O. 1967. The theory of island biogeography. Princeton, NJ: Princeton University Press.
Mailliot
S.,
Mattioli
E.,
Bartolini
A.,
Baudin
F.,
Pittet
B.
&
Guex
J. 2009.
Late Pliensbachian–Early Toarcian (Early Jurassic) environmental changes in an epicontinental basin of NW Europe (Causses area, central France): a micropaleontological and geochemical approach. Palaeogeography, Palaeoclimatology, Palaeoecology 273, 346–364.
Publisher Full Text
McArthur
J.M.,
Donovan
D.T.,
Thirvall
M.F.,
Fouke
B.W.
&
Mattey
D. 2000.
Strontium isotope of the Early Toarcian (Jurassic) oceanic anoxic event, the duration of ammonite biozones and belemnite palaeotemperatures. Earth and Planetary Science Letters 179, 269–285.
Publisher Full Text
McGann
M.
&
Sloan
D. 1999. Benthic foraminifers in the regional monitoring program's San Francisco estuary samples: Richmond, San Francisco Estuary Institute. In: San Francisco Estuary Regional Monitoring Program for Trace Substances. 1997 annual report. Pp. 249–258.
Richmond, CA: San Francisco Estuary Institute.
Morten
S.D.
&
Twitchett
R.J. 2009.
Fluctuations in the body size of marine invertebrates through the Pliensbachian–Toarcian extinction event. Palaeogeography, Palaeoclimatology, Palaeoecology 284, 29–38.
Publisher Full Text
Murray
J.W.,
Alve
E.
&
Jones
B.W. 2011.
A new look at modern agglutinated benthic foraminiferal morphogroups: their value in palaeoecological interpretation. Palaeogeography, Palaeoclimatology, Palaeoecology 309, 229–241.
Publisher Full Text
Nagy
J. 1992.
Environmental significance of foraminiferal morphogroups in Jurassic North Sea deltas. Palaeogeography, Palaeoclimatology, Palaeoecology 95, 111–134.
Publisher Full Text
Nagy
J.
&
Berge
S.H. 2008.
Micropalaeontological evidence of brackish water conditions during deposition of the Knorringfjellet Formation, Late Triassic–Early Jurassic, Spitsbergen. Polar Research 27, 413–427.
Publisher Full Text
Nagy
J.,
Hess
S.
&
Alve
E. 2010.
Environmental significance of foraminiferal assemblages dominated by small-sized Ammodiscus and Trochammina in Triassic and Jurassic delta-influenced deposits. Earth-Science Reviews 99, 31–49.
Publisher Full Text
Nagy
J.
&
Johansen
H.O. 1991.
Delta-influenced foraminiferal assemblages from the Jurassic (Toarcian–Bajocian) of the northern North Sea. Micropaleontology 37, 1–7.
Publisher Full Text
Nagy
J.,
Reolid
M.
&
Rodríguez-Tovar
F.J. 2009.
Foraminiferal morphogroups in dysoxic shelf deposits from the Jurassic of Spitsbergen. Polar Research 28, 214–221.
Publisher Full Text
Nikitenko
B.L. 2008.
The Early Jurassic to Aalenian paleobiogeography of the Arctic Realm: implications of microbenthos (foraminifers and ostracodes): stratigraphy. Geological Correlation 16, 59–80.
Nikitenko
B.L. 2009. Jurassic stratigraphy, palaeobiogeography and biofacies of Siberia on microfauna (foraminifers and ostracodes).
Novosibirsk: Parallel.
Nikitenko
B.L.
&
Mickey
M.B. 2004.
Foraminifera and ostracodes across the Pliensbachian–Toarcian boundary in the Arctic Realm (stratigraphy, paleobiogeography and biofaces). Journal Geological Society Special Publication 230, 137–174.
Publisher Full Text
Nikitenko
B.L.,
Reolid
M.
&
Glinskikh
L. 2013.
Ecostratigraphy of benthic foraminifera for interpreting Arctic record of Early Toarcian biotic crisis (northern Siberia, Russia). Palaeogeography, Palaeoclimatology, Palaeoecology 376, 200–212.
Publisher Full Text
Nocchi
M.
&
Bartolini
A. 1994.
Investigations on Late Domerian–Early Toarcian Lagenina and Glomospirella assemblages in the Umbria Marche Basin (central Italy). Geobios M.S. 17, 689–699.
Publisher Full Text
Nyong
E.E.
&
Ramanathan
R. 1985.
A record of oxygen-deficient paleoenvironments in the Cretaceous of the Calabar Flank, SE Nigeria. Journal of African Earth Sciences 3, 455–460.
Publisher Full Text
Price
G.D. 1999.
The evidence and implication of polar ice during the Mesozoic. Earth-Science Reviews 48, 183–210.
Publisher Full Text
Reolid
M.,
Chakiri
S.
&
Bejjaji
Z. 2013.
Adaptative strategies of the Toarcian benthic foraminiferal assemblages from the Middle Atlas (Morocco): palaeoecological implications. Journal of African Earth Sciences 84, 1–12.
Publisher Full Text
Reolid
M.
&
Nagy
J. 2008.
Jurassic transgressive–regressive cycles in carbonate and siliciclastic shelf facies: different response of foraminiferal assemblage trends to sea-level changes. Grzybowski Foundation Special Publication 13, 199–213.
Reolid
M.,
Nagy
J.
&
Rodríguez-Tovar
F.J. 2010.
Ecostratigraphic trends of Jurassic agglutinated foraminiferal assemblages as a response to sea-level changes in shelf deposits of Svalbard (Norway). Palaeogeography, Palaeoclimatology, Palaeoecology 293, 184–196.
Publisher Full Text
Reolid
M.,
Nagy
J.,
Rodríguez-Tovar
F.J.
&
Olóriz
F. 2008.
Foraminiferal assemblages as palaeoenvironmental bioindicators in Late Jurassic epicontinental platforms: relation with trophic conditions. Acta Palaeontologica Polonica 53, 706–722.
Publisher Full Text
Reolid
M.,
Rodríguez-Tovar
F.J.,
Marok
A.
&
Sebane
A. 2012.
The Toarcian Oceanic Anoxic Event in the Western Saharan Atlas, Algeria (North African Paleomargin): role of anoxia and productivity. Geological Society of America Bulletin 124, 1646–1664.
Publisher Full Text
Reolid
M.,
Rodríguez-Tovar
F.J.
&
Nagy
J. 2012.
Ecological replacement of Valanginian agglutinated foraminifera during a maximum flooding event in the Boreal realm (Spitsbergen). Cretaceous Research 33, 196–204.
Publisher Full Text
Reolid
M.,
Rodríguez-Tovar
F.J.,
Nagy
J.
&
Olóriz
F. 2008.
Benthic foraminiferal morphogroups of mid to outer shelf environments of the Late Jurassic (Prebetic Zone, southern Spain): characterisation of biofacies and environmental significance. Palaeogeography, Palaeoclimatology, Palaeoecology 261, 280–299.
Publisher Full Text
Reolid
M.,
Sebane
A.,
Rodríguez-Tovar
F.J.
&
Marok
A. 2012.
Foraminiferal morphogroups as a tool to approach the Toarcian Anoxic Event in the Western Saharan Atlas (Algeria). Palaeogeography, Palaeoclimatology, Palaeoecology 323–325, 87–99.
Publisher Full Text
Rey
J.,
Bonnet
L.,
Cubaynes
R.,
Qajoun
A.
&
Ruget
C. 1994.
Sequence stratigraphy and biological signals: statistical studies of benthic foraminifera from Liassic series. Palaeogeography, Palaeoclimatology, Palaeoecology 111, 149–171.
Publisher Full Text
Röhl
H.J.,
Schmid-Röhl
A.,
Oschmann
W.,
Frimmel
A.
&
Schwark
L. 2001.
The Posidonian Shale (Lower Toarcian) of SW-Germany: an oxygen-depleted ecosystem controlled by sea level and paleoclimate. Palaeogeography, Palaeoclimatology, Palaeoecology 165, 27–52.
Publisher Full Text
Rosales
I.,
Quesada
S.
&
Robles
S. 2004.
Paleotemperature variations of Early Jurassic seawater recorded in geochemical trends of belemnites from the Basque–Cantabrian basin, northern Spain. Palaeogeography, Palaeoclimatology, Palaeoecology 203, 253–275.
Publisher Full Text
Scotese
C.R.
2011. Paleogeographic reconstructions of the circum-Arctic region since the Late Jurassic. Search and Discovery, article no. 30193.
Setoyama
E.,
Radmacher
W.,
Kaminski
M.A.
&
Tyszka
J. 2013.
Foraminiferal and palynological biostratigraphy and biofacies from a Santonian–Campanian submarine dan system in the Vøring Basin (offshore Norway). Marine and Petroleum Geology 43, 396–408.
Publisher Full Text
Sjoerdsma
P.G.
&
Van der Zwaan
G.J. 1992.
Simulating the effect of changing organic flux and oxygen content on the distribution of benthic foraminifera. Marine Micropaleontology 19, 163–180.
Publisher Full Text
Song
H.,
Tong
J.
&
Chen
Z.Q. 2011.
Evolutionary dynamics of the Permian–Triassic foraminifer size: evidence for Lilliput effect in the end-Permian mass extinction and its aftermath. Palaeogeography, Palaeoclimatology, Palaeoecology 308, 98–110.
Publisher Full Text
Soua
M.,
Zaghbin-Turki
D.,
Ben Jemia
H.,
Smaoui
J.
&
Boukadi
A. 2011.
Geochemical record of the Cenomanian–Turonian Anoxic Event in Tunisia: is it correlative and isochronous to the biotic signal? Acta Geologica Sinica-English Edition 85, 1310–1335.
Publisher Full Text
Suan
G.,
Mattioli
E.,
Pittet
B.,
Lécuyer
C.,
Suchéras-Marx
B.,
Duarte
L.V.,
Philippe
M.,
Reggiani
M.L.
&
Martineau
F. 2010.
Secular environmental precursors of Early Toarcian (Jurassic) extreme climate changes. Earth and Planetary Science Letters 290, 448–458.
Publisher Full Text
Suan
G.,
Nikitenko
B.L.,
Rogov
M.A.,
Baudin
F.,
Spangenberg
J.E.,
Knyazev
V.G.,
Glinskikh
L.A.,
Goryacheva
A.A.,
Adatte
T.,
Riding
J.B.,
Föllmi
K.B.,
Pittet
B.,
Mattioli
E.
&
Lécuyer
C. 2011.
Polar record of Early Jurassic massive carbon injection. Earth and Planetary Science Letters 312, 102–113.
Publisher Full Text
Svensen
H.,
Planke
S.,
Chevallier
L.,
Malthe-Sorenssen
A.,
Corfu
F.
&
Jamtveit
B. 2007.
Hydrothermal venting of greenhouse gases triggering Early Jurassic global warming. Earth and Planetary Science Letters 290, 448–458.
Thurman
H.V.
&
Trujillo
A.P. 2004. Introductory oceanography. Upper Saddle River, NJ: Prentice Hall.
Torsvik
T.H.,
Van der Voo
R.,
Preeden
U.,
MacNiocaill
C.,
Steinberger
B.,
Doubrovine
P.V.,
van Hinsbergen
D.J.J.,
Domeier
M.,
Gaina
C.,
Tohver
E.,
Meert
J.G.,
McCausland
P.J.A.
&
Cocks
L.R.M. 2012.
Phanerozoic polar wander, palaeogeography and dynamics. Earth-Science Reviews 114, 325–368.
Publisher Full Text
Tsujimoto
A.,
Nomura
R.,
Yasuhara
M.,
Yamazaki
H.
&
Yoshikawa
S. 2006.
Impact of eutrophication on shallow marine benthic foraminifers over the last 150 years in Osaka Bay, Japan. Marine Micropaleontology 60, 258–268.
Publisher Full Text
Twitchett
R.J. 2007.
The Lilliput effect in the aftermath of the end-Permian extinction event. Palaeogeography, Palaeoclimatology, Palaeoecology 252, 132–144.
Publisher Full Text
Tyszka
J. 1994.
Response of Middle Jurassic benthic foraminiferal morphogroups to dysoxic/anoxic conditions in the Pieniny Klippen Basin, Polish Carpathians. Palaeogeography, Palaeoclimatology, Palaeoecology 110, 55–81.
Publisher Full Text
Tyszka
J. 2009.
Foraminiferal response to seasonality modulated by orbital cycles in the Cretaceous mid-latitudes: the Albian record from the Lower Saxony Basin. Palaeogeography, Palaeoclimatology, Palaeoecology 276, 148–159.
Publisher Full Text
Tyszka
J. 2010. Seasonal opportunists: in fossilio experiment on Albian foraminifera. Taxonomic challenges and innovations. In: The Micropalaeontological Society's Foraminifera and Nannofossil Groups’ Joint Spring Meeting. 28 June, The Natural History Museum, London. Pp. 9–10.
London: Micropalaeontological Society.
Valentine
J.W. 1973. Evolutionary ecology of the marine biosphere. Englewood Cliffs, NJ: Prentice Hall.
Van de Schootbrugge
B.,
McArthur
J.M.,
Bailey
T.R.,
Rosenthal
Y.,
Wright
J.D.
&
Miller
K.G.
2005. Toarcian oceanic anoxic event: an assessment of global causes using belemnite C isotope records. Paleoecanography
20, PA3008, doi: 10.1029/2004PA001102.
Publisher Full Text
Van der Zwaan
G.J.,
Duijnstee
I.A.P.,
Den Dulk
M.,
Ernst
S.R.,
Jannink
N.T.
&
Kouwenhoven
T.J. 1999.
Benthic foraminifers: proxies or problem? A review of paleoecological concepts. Earth-Science Reviews 46, 213–236.
Publisher Full Text
Wignall
P.B.,
Newton
R.J.
&
Little
C.T.S. 2005.
The timing of paleoenvironmental change and cause-and-effect relationships during the Early Jurassic mass extinction in Europe. American Journal of Sciences 305, 1014–1032.