RESEARCH/REVIEW ARTICLE
Persistent organic pollutants in biota samples collected during the Ymer-80 expedition to the Arctic
Henrik Kylin,1,2,3 Johan Hammar,4,5 Jacques Mowrer,1 Henk Bouwman,6 Carl Edelstam,5 Mats Olsson5
& Sören Jensen1
1 Department of Environmental Science and Analytical Chemistry, Stockholm University, SE-106 91 Stockholm, Sweden
2 Norwegian Institute for Air Research, Fram Centre, NO-9296 Tromsø, Norway
3 Department of Thematic Studies—Environmental Change, Linköping University, SE-581 83 Linköping, Sweden
4 Institute of Freshwater Research, Department for Aquatic Resources, Swedish University of Agricultural Sciences, SE-178 93 Drottningholm, Sweden
5 Natural History Museum, P.O. Box 50007, SE-104 05 Stockholm, Sweden
6 Research Unit: Environmental Sciences and Development, North-West University, P. Bag X6001, Potchefstroom 2520, South Africa
Abstract
During the 1980 expedition to the Arctic with the icebreaker Ymer, a number of vertebrate species were sampled for determination of persistent organic pollutants. Samples of Arctic char (Salvelinus alpinus, n=34), glaucous gull (Larus hyperboreus, n=8), common eider (Somateria mollissima, n=10), Brünnich’s guillemot (Uria lomvia, n=9), ringed seal (Pusa hispida, n=2) and polar bear (Ursus maritimus, n=2) were collected. With the exception of Brünnich’s guillemot, there was a marked contamination difference of birds from western as compared to eastern/northern Svalbard. Samples in the west contained a larger number of polychlorinated biphenyl (PCB) congeners and also polychlorinated terphenyls, indicating local sources. Brünnich’s guillemots had similar pollutant concentrations in the west and east/north; possibly younger birds were sampled in the west. In Arctic char, pollutant profiles from lake Linnévatn (n=5), the lake closest to the main economic activities in Svalbard, were similar to profiles in Arctic char from the Shetland Islands (n=5), but differed from lakes to the north and east in Svalbard (n=30). Arctic char samples had higher concentrations of hexachlorocyclohexanes (HCHs) than the marine species of birds and mammals, possibly due to accumulation via snowmelt. Compared to the Baltic Sea, comparable species collected in Svalbard had lower concentrations of PCB and dichlorodiphenyltrichloroethane (DDT), but similar concentrations indicating long-range transport of hexachlorobenzene, HCHs and cyclodiene pesticides. In samples collected in Svalbard in 1971, the concentrations of PCB and DDT in Brünnich’s guillemot (n=7), glaucous gull (n=2) and polar bear (n=2) were similar to the concentrations found in 1980.
Keywords
Polar bear; ringed seal; glaucous gull; Brünnich’s guillemot; common eider; Arctic char.
Citation: Polar Research 2015, 34, 21129, http://dx.doi.org/10.3402/polar.v34.21129
Copyright: © 2015 H. Kylin et al. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License, permitting all non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Published: 26 October 2015
Correspondence to: Henrik Kylin, Department of Thematic Studies—Environmental Change, Linköping University, SE-581 83 Linköping, Sweden.
E-mail: henrik.kylin@liu.se
To access the supplementary material for this article, please see supplementary files under Article Tools online.
Contamination of the Arctic with persistent organic pollutants (POPs) gained interest in the 1980s when it was realized that northern indigenous peoples may carry high body burdens of these anthropogenic contaminants (Dewailly et al. 1989; de March et al. 1998). This triggered research to measure various POPs in biotic and abiotic samples from the Arctic with the aim to understand and model their global transport and fate (Wania & Mackay 1996). As a consequence, the Arctic Monitoring and Assessment Programme, an international working group under the Arctic Council, was established in 1991 to aid policymakers and implement the Arctic Environmental Protection Strategy (AMAP 2011).
In 1980, prior to the increased interest in organic contaminants in the Arctic, the Swedish icebreaker HMS Ymer performed an expedition to the eastern Arctic Ocean, the Ymer-80 expedition (Schytt 1983; Hoppe et al. 1987). The broad research programme included sampling of wildlife for the Swedish Museum of Natural History (SMNH). SMNH was, and is, a hub for the Swedish national environmental monitoring programme (SNEMP) for POPs in biota. The SNEMP for POPs was initiated at the end of the 1960s and includes, for example, sampling of fish in the sub-Arctic lakes of northern Sweden and fish and guillemot eggs from the central Baltic Sea. Consequently, some samples collected during Ymer-80 were analysed for the presence of POPs to compare the contaminant concentrations in the Arctic with Swedish limnic and marine environments. However, the results of this early investigation of the contamination situation at Svalbard were never published except as a popular science cruise report in Swedish (Edelstam et al. 1987), presenting only partial data. Here we present the complete data set, as far as it has been possible to reconstruct, of POP concentrations in the samples collected during Ymer-80 and complementary samples collected in 1971, 1979 and 1981. We also present data from seal samples collected in 1983–84, which have not been properly published. It is outside the scope of this paper to analyse time trends for all the species included and space would not allow it. Such time-trend analyses will be the subject of subsequent papers.
Material and methods
Muscle samples of Arctic char (Salvelinus alpinus; anadromous, resident and landlocked populations), common eider (Somateria mollissima), Brünnich’s guillemot (Uria lomvia), glaucous gull (Larus hyperboreus) and polar bear (Ursus maritimus), and blubber samples of ringed seal (Pusa hispida) were obtained from specimens collected for the SMNH during the Ymer-80. Muscle samples of Brünnich’s guillemots and polar bears from 1971 were donated by Norwegian authorities. Sampling locations are shown in Fig. 1 and sample details are given in Supplementary Tables S1 and S2.
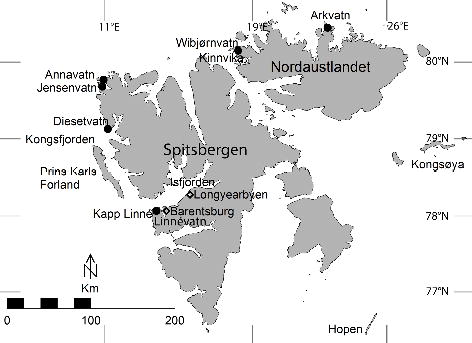
Fig. 1
Sampling sites in Svalbard. Arctic char (Salvelinus alpinus) were sampled in the lakes indicated on the map. In western Svalbard, glaucous gull (Larus hyperboreus) and common eider (Somateria mollissima) were sampled in Isfjorden, mainly around Kapp Linné, and Brünnich’s guillemot (Uria lomvia) on Prins Karls Forland. In the north and east, eiders were sampled around Kinnvika on Nordaustlandet, gulls at Kinnvika and Kongsøya, and guillemots on Kongsøya and Hopen.
The total list of analytes include hexachlorobenzene (HCB); α-, β-, γ- and δ- hexachlorocyclohexane (HCH); p,p′- dichlorodiphenyltrichloroethane (DDT); o,p′-DDT; p,p′- dichlorodiphenyldichloroethane (DDD); o,p′-DDD; p,p′-dichlorodiphenyldichloroethylene (DDE);
o,p′-DDE; methoxychlor; toxaphene; aldrin; dieldrin; endrin; oxy-, α- and γ-chlordane; α- and γ-chlordene; cis- and trans-nonachlor; heptachlor; heptachlor epoxide; polychlorinated biphenyl (PCB) and polychlorinated terphenyls (PCT). For formal chemical names see Supplementary Table S3.
Analyses were performed in 1983–84 at which time data were recorded in hand-written files. The data sets were digitalized in 2008–2012. As far as possible, missing data were recalculated based on saved chromatograms and integrator data. Limits of detection (LOD) and quantification (LOQ) were not possible to reconstruct in detail. For simplicity, the LOQ was set to 0.01 µg g−1 lipid for all analytes and the LOD to 0.003 µg g−1 lipid.
The analytical procedure (see the Supplementary file for a brief description), including quality control/quality assurance, was state-of-the-art at the time of analysis. The quantifications were performed by fused-silica capillary column gas chromatography with either electron capture detector or mass spectrometer to quantify individual compounds and congeners. Most of the results presented here should be comparable to more recent investigations. However, PCT and toxaphene data should be treated with caution. Individual PCT or toxaphene congeners were not available as standards. The data are included here to evaluate the spatial trends within the Svalbard Archipelago, not for comparison with other studies.
For comparison between data from 1971 and 1980 (Supplementary Table S8), the 1980 data were recalculated based on intercalibration of different quantification methods used over time in the SNEMP. Additional unpublished data from samples collected 1983 and 1984 in an investigation commissioned by the Norwegian Environmental Protection Agency are included for comparison (Supplementary Table S9). See further discussion in the Supplementary file for details.
Principal component analysis (PCA) was used for pollutant pattern comparisons between samples and groups of samples, using MjM Software PC-ORD version 6.07. Details of data treatment are given in the Supplementary file.
Results and discussion
The sampling programme of Ymer-80 was planned both to obtain information on POP contamination where few previous sampling campaigns had taken place and to gain information from a “pristine background area” useful for comparisons with SNEMP data from the Swedish environment (Edelstam et al. 1987). The vertebrates targeted for sampling, therefore, either occur or have close relatives in Sweden. Arctic char, common eider and ringed seal all occur in Sweden, while the Brünnich’s guillemot of Svalbard is closely related to the common guillemot (Uria aalge) in the Baltic. As complement to these species, specimens of glaucous gull and polar bear were collected as representing the high trophic levels in the Arctic.
The sampling was also intended to distinguish difference between western and northern/eastern Svalbard. Economic activities in Svalbard were and are concentrated chiefly around Isfjorden and Van Mijenfjorden in the west, and the west potentially also is more exposed to contaminants arriving with ocean currents. The hypothesis was, therefore, that western Svalbard should have higher contamination levels than northern/eastern Svalbard.
Data are presented below and in the Supplementary file on a lipid mass basis. Information enabling conversion to a fresh mass basis is given in Supplementary Table S1.
Birds
Summary data on the contaminant concentrations are given in Table 1, with details in Supplementary Table S4. The contaminant concentrations were distinctly different in birds from western compared to northern/eastern Svalbard. In common eider and glaucous gull, perhaps the most obvious difference was the presence of PCT in samples from western Svalbard. PCT are three-ringed analogues to PCB (which has two rings). The two had similar uses, but PCT formulations were generally used at higher temperatures (de Boer 2000). Data on PCT contamination is scanty worldwide, the presence of PCT in only one area of Svalbard indicates that western Svalbard was subject to contamination from local economic activities. All samples with PCT residues were collected on or close to Kapp Linné, with both mining operations (Barentsburg) and a main telecommunications facility. However, exactly which activities in the area required the use of PCT is impossible to say. Nor is it possible to know if any PCT was still used at the time of sampling, or if the PCT residues were due to historical use. We cannot exclude that the birds also picked up contaminants in their wintering areas, but the number of species (including Arctic char, see below) contaminated with PCT in western Svalbard makes it unlikely that the main source of PCT was the wintering areas.
Table 1 Summary of organochlorine concentrations (µg g−1 lipid) in birds and mammals collected during the Ymer-80 expedition in the northern and eastern (N/E) and western (W) parts of Svalbard. Concentrations of individual analytes in individual samples are presented in Supplementary Tables S4a and S4b.
| |
|
HCB
Mean
Median
Range |
Σ31PCB
Mean
Median
Range |
Σ6PCBa
Mean
Median
Range
|
ΣPCT
Mean
Median
Range |
Oxychlordane
Mean
Median
Range |
p,p′-DDE
Mean
Median
Range |
ΣDDTb
Mean
Median
Range
|
Lipid%
Mean
Median
Range |
| Brünnich’s guillemot |
N/E (n=4) |
1.1 |
11 |
7.9 |
NDc
|
0.28 |
6.6 |
6.7 |
3.0 |
| |
|
0.55 |
10 |
7.0 |
— |
0.13 |
5.8 |
5.8 |
2.7 |
| |
|
0.35–3.0 |
4.7–20 |
3.2–14 |
— |
0.11–0.28 |
3.1–12 |
3.1–12 |
2.0–4.6 |
| |
W (n=5) |
0.39 |
3.0 |
2.2 |
ND |
0.14 |
1.8 |
1.8 |
2.8 |
| |
|
0.24 |
1.4 |
1.2 |
— |
0.15 |
1.4 |
1.4 |
2.9 |
| |
|
0.13–1.1 |
0.74–4.3 |
0.57–5.1 |
— |
0.02–0.28 |
0.51–3.7 |
0.98–3.7 |
2.3–3.0 |
| Glaucous gull |
N/E (n=2) |
0.88 |
52 |
43 |
ND |
0.92 |
15 |
15 |
3.7 |
| |
|
— |
— |
— |
— |
— |
— |
— |
— |
| |
|
0.50–1.3 |
5.5–98 |
4.0–83 |
— |
0.29–1.6 |
3.1–27 |
3.2–27 |
3.1–4.4 |
| |
W (n=6)d
|
2.7 |
78 |
64 |
23 |
1.9 |
29 |
30 |
6.7 |
| |
|
2.7 |
65 |
50 |
20 |
1.7 |
29 |
30 |
|
| |
|
1.2–5.3 |
38–150 |
29–120 |
14–35 |
1.3–2.7 |
20–38 |
20–39 |
4.4–8.6 |
| Common eider |
N/E (n=5) |
0.11 |
1.0 |
0.75 |
ND |
0.06 |
0.60 |
0.64 |
2.8 |
| |
|
0.11 |
0.80 |
0.58 |
— |
0.05 |
0.46 |
0.49 |
3.0 |
| |
|
0.07–0.18 |
0.41–1.9 |
0.28–1.5 |
— |
tre–0.10 |
0.24–0.98 |
0.26–1.1 |
1.8–4.4 |
| |
W (n=5) |
0.10 |
3.1 |
1.1 |
17 |
0.75 |
0.44 |
0.48 |
2.8 |
| |
|
0.09 |
2.9 |
1.0 |
15 |
0.83 |
0.33 |
1.0 |
3.1 |
| |
|
0.06–0.15 |
2.3–4.3 |
0.65–2.0 |
8.0–29 |
0.10–1.4 |
0.17–1.0 |
0.20–1.1 |
2.0–3.4 |
| Ringed seal |
N/E (n=2) |
0.02 |
2.4 |
1.1 |
ND |
0.22 |
0.07 |
0.8 |
93 |
| |
|
— |
— |
— |
— |
— |
— |
— |
— |
| |
|
0.01–0.02 |
1.6–3.2 |
0.65–1.6 |
— |
0.09–0.34 |
0.06–0.08 |
0.61–0.99 |
90–96 |
| Polar bear |
N/E (n=2) |
— |
140 |
130 |
ND |
4.4 |
— |
— |
1.0 |
| |
|
— |
— |
— |
— |
— |
— |
— |
— |
| |
|
ND–0.06 |
3.5–280 |
3.2–260 |
— |
0.11–8.6 |
Mf–33 |
M–0.40 |
0.77–1.3 |
| aSum of CB-99, −118, −138, −153, −170 and −180. Provided here for comparisons with data on glaucous gulls in Verreault et al. (2010). bSum of o,p′-DDE, p,p′-DDE, o,p′-DDD, p,p′-DDD, o,p′-DDT and p,p′-DDT. cND - not detected. dn=5 for ΣPCT, oxychlordane and lipid%. etr - trace (0.003 μg g−1 lipid≤tr<0.01 μg g−1 lipid). fM - missing data. |
In common eider, the concentrations of ΣPCB were higher and the congener pattern more complex in western than in northern/eastern Svalbard, which strengthens the suspicion of a local source of contaminants. Mehlum & Daelemans (1995), too, suggested the presence of a local source of PCB in western Svalbard in the 1980s. However, the source is not necessarily activities on Kapp Linné only; activities in Longyearbyen and Sveagruva may also contribute contaminants to western Svalbard. For glaucous gull, the number of samples from the northern/eastern part of the archipelago is too low for any relevant comparison of PCB as the concentrations in these two samples vary with two orders of magnitude. It is noteworthy, though, that the glaucous gulls from western Svalbard contained substantially more chlordanes than those from the northern/eastern parts. But this is not reflected for common eider, in which the concentration differences may even be the opposite.
In contrast to the other two bird species, POP concentrations in Brünnich’s guillemot did not differ much between western and northern and eastern Svalbard. This is somewhat surprising; more pronounced differences were expected as fish-eating guillemots feed at a higher trophic level than mussel-eating eider. Common and Brünnich’s guillemots have similar feeding ecologies and it was expected that their body burdens relative to common eiders would be similar in any given area. In the Baltic, common guillemots generally had three to five times higher concentrations of PCB than common eiders (Edelstam et al. 1987), and a similar ratio was found between Brünnich’s guillemots and common eiders in northern/eastern Svalbard, but not the western part. A possible explanation for the similar concentrations of POPs in guillemots and eiders from western Svalbard is that juvenile guillemots were sampled in the west. There was no way of ascertaining the age of the birds at the time of analysis as the collection of these samples was done separately by Norwegian staff and only the breast muscle was sent from the collector to the SMNH. An alternative explanation is that while common eider feed on locally contaminated stationary resources close to shore, guillemots forage on less contaminated pelagic fish (Edelstam et al. 1987). A third explanation is that the guillemots have different food choices in the western and northern/eastern parts of Svalbard. An investigation of the stomach content of some of the guillemots collected during Ymer-80 indicated large individual differences in food choice. Some individuals had fed on fish, while the stomach contents of others consisted of >99% crustaceans (amphipods and mysids, J. Hammar pers. obs.), suggesting that the differences in contaminant levels could be explained by individual guillemots feeding at different trophic levels. However, we cannot presently tie individual bird samples to specific a specific trophic level; determination of stable carbon and nitrogen isotope ratios (δ13C and d15N) would be helpful.
The differences in the pollution patterns between western and northern/eastern Svalbard are demonstrated by a PCA of the relativized data in all the bird species (Fig. 2). All the northern/eastern samples form convex hulls far to the left on the first axis (closely parallel with the p,p’-DDE vector), separating them from the samples from western Svalbard (closely paralleled with the vectors for ΣPCT and PCB congeners 44, 52, 92, 95, 97 and 101 [vector numbers 4b, 4a, 5b, 5a, 5f and 5d, respectively]). It is also noteworthy that in both geographical categories the eiders separate from the guillemots and gulls, which overlap with each other within each geographical category. Therefore, even if there is no obvious difference in the concentrations in eiders and guillemots, the fact that they feed at different trophic levels gives rise to different contaminant patterns. This is emphasized by the overlap of the guillemots and glaucous gulls in the PCA plot; both species are at least partially fish-eaters.
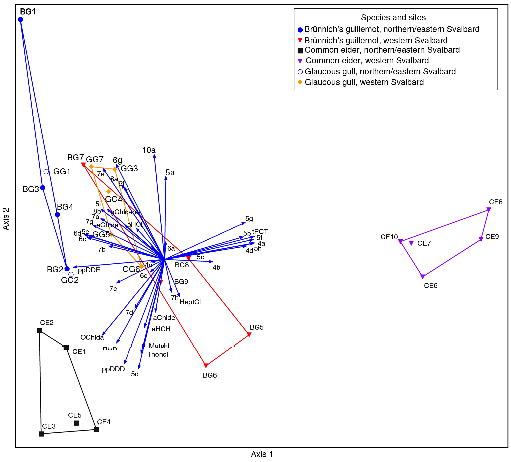
Fig. 2
Principal component analysis of relativized (see the Supplementary file for details) contaminant concentrations in birds. Vector numbers refer to individual polychlorinated biphenyl (PCB) congeners (see Supplementary Table S3). Axis 1 explains 76% and Axis 2 20% of the total variation. Vector numbers refer to individual PCB congeners; the numeral refers to the number of chlorines in the molecule, see Supplementary Table S3b for full explanation. Samples from different locations are presented by convex hulls. There were only two samples of glaucous gull from northern/eastern Svalbard and these are represented by their coordinate points only. Identities of individual samples are given in Supplementary Table S4.
Glaucous gulls have been used to monitor bioaccumulating environmental pollutants in Svalbard (Verreault et al. 2010), and common eider and Brünnich’s guillemots have been included occasionally (Mehlum & Daelemans 1995; Sagerup et al. 2009). To compare the Ymer-80 data with the data from other studies, we calculated a Σ6PCB (Table 1). Generally, the contaminant concentrations from Ymer-80 compare well with concentrations reported for the respective species during the 1980s.
Table 2 Summary of organochlorine concentrations (µg g−1 lipid) in Arctic char collected during the Ymer-80 expedition. Concentrations of individual analytes in individual samples are presented in Supplementary Table S5.
| |
|
HCB
Mean
Median
Range |
Σ31PCB
Mean
Median
Range |
ΣHCH
Mean
Median
Range |
p,p′-DDE
Mean
Median
Range |
Lipid%
Mean
Median
Range |
| Linnévatn |
Smolt (n=3) |
0.07 |
5.2 |
1.5 |
0.56 |
1.2 |
| |
|
0.07 |
4.2 |
1.3 |
0.38 |
1.0 |
| |
|
0.05–0.10 |
1.6–9.7 |
0.64–2.5 |
0.38–1.0 |
0.37–2.2 |
| |
Resident (n=3) |
0.04 |
0.71 |
1.9 |
0.08 |
1.6 |
| |
|
0.05 |
0.73 |
1.9 |
0.07 |
1.5 |
| |
|
0.02–0.05 |
0.47–0.94 |
1.4–2.5 |
0.04–0.11 |
0.99–2.4 |
| |
All (n=6) |
0.06 |
2.9 |
1.7 |
0.32 |
1.4 |
| |
|
0.05 |
1.27 |
1.6 |
0.21 |
1.3 |
| |
|
0.02–0.10 |
0.47–9.7 |
0.24–4.4 |
0.04–1.0 |
0.37–2.4 |
| Diesetvatn |
Anadromous (n=2) |
0.09 |
0.31 |
3.8 |
0.07 |
3.7 |
| |
|
— |
— |
— |
— |
— |
| |
|
0.08–0.09 |
0.27–0.34 |
3.2–4.5 |
0.14–0.15 |
3.0–4.3 |
| Jensenvatn |
Resident (n=6) |
0.14 |
6.7 |
6.1 |
1.2 |
5.1 |
| |
|
0.13 |
7.0 |
5.3 |
1.2 |
4.4 |
| |
|
0.11–0.19 |
2.1–10 |
2.9–13 |
0.20–2.3 |
2.0–12 |
| Annavatn |
Resident (n=5) |
0.19 |
7.5 |
2.7 |
1.7 |
2.3 |
| |
|
0.19 |
7.4 |
2.7 |
1.5 |
2.4 |
| |
|
0.13–0.22 |
4.0–11 |
2.1–3.4 |
0.64–2.9 |
1.7–3.0 |
| Wibjørnvatn |
Resident (n=7) |
0.17 |
1.8 |
3.8 |
0.38 |
3.6 |
| |
|
0.17 |
1.5 |
4.0 |
0.30 |
3.8 |
| |
|
0.14–0.20 |
1.0–3.3 |
1.8–6.2 |
0.19–0.84 |
1.6–6.0 |
| Arkvatn |
Smolt? (n=5) |
0.20 |
1.3 |
2.9 |
2.6 |
2.5 |
| |
|
0.20 |
1.2 |
3.2 |
2.5 |
2.8 |
| |
|
0.16–0.22 |
0.85–1.9 |
2.0–3.6 |
1.1–4.1 |
1.7–3.2 |
| |
Resident? (n=3) |
0.18 |
0.59 |
4.0 |
0.43 |
3.6 |
| |
|
0.19 |
0.61 |
3.6 |
0.44 |
3.2 |
| |
|
0.15–0.20 |
0.54–0.61 |
3.5–5.0 |
0.19–0.66 |
3.1–4.6 |
| |
All (n=8) |
0.19 |
1.0 |
3.3 |
1.8 |
2.9 |
| |
|
0.20 |
0.93 |
3.3 |
1.5 |
2.9 |
| |
|
0.15–0.22 |
0.54–1.9 |
2.0–5.0 |
0.19–4.1 |
1.6–4.6 |
| Girlsta Loch |
Resident (n=5) |
0.09 |
4.7 |
1.1 |
1.4 |
0.88 |
| |
|
0.09 |
3.4 |
1.0 |
0.77 |
0.82 |
| |
|
0.08–0.09 |
2.1–9.6 |
1.0–1.2 |
0.37–3.5 |
0.78–1.0 |
Samples from two specimens of common guillemot from the central Baltic Sea were included among the Svalbard samples as reference (Supplementary Table S7). The ΣPCB and ΣDDT concentrations were substantially higher in these samples than in samples of Brünnich’s guillemot from Svalbard, while the concentrations of HCB, ΣHCH and oxychlordane are similar or even higher in Svalbard than in the Baltic. This suggests that local sources of PCB and DDT affected the Baltic more than Svalbard, but that the other contaminants may have reached both areas mainly by long-range atmospheric transport (Edelstam et al. 1987).
Other data on concentrations of POPs in birds from western Svalbard from the early 1980s have been published (Norheim & Kjos-Hanssen 1984; Carlberg & Bøler 1985), but the concentrations are difficult to compare with the concentration reported here for the Ymer-80 samples as the quantifications were done using an old packed-column gas chromatography method. See further discussion in the Supplementary file.
Mammals
Summary data on contaminant concentrations in ringed seals and polar bears are given in Table 1, with details in Supplementary Table S4. Too few specimens were analysed to allow far-reaching conclusions from this material alone; the data are presented here to allow comparisons with other studies.
Both polar bears were found north-east of Svalbard. As expected, an emaciated individual had the highest concentrations of all analytes (except HCB), but it is noteworthy that the number of PCB congeners detected was almost the same in both specimens. The additional congeners in the emaciated individual were present at much lower concentrations than the dominant congeners. Polar bears efficiently degrade most PCB congeners (Muir et al. 1988; Norstrom et al. 1988). The DDE and PCB concentrations in the non-emaciated specimen was similar to those reported for polar bears from many areas of the Arctic in the period 1996–2002 (Verreault et al. 2005), while the oxychlordane concentrations was slightly lower than reported for Svalbard in that study.
Both ringed seals were males. The contaminant patterns were fairly similar although the concentrations of PCBs and DDTs were about twice as high in the seal from north-eastern Svalbard than the seal from Kongsfjorden in western Svalbard, while the chlordane concentrations were about five times higher. The higher concentrations were accompanied by a larger number of detected congeners.
Additional data on five ringed seals and two bearded seals (Erignathus barbatus) were reported by Carlberg & Bøler (1985). These samples were analyses in the same laboratory, by the same staff, using the same methods as the Ymer-80 samples. However, for reasons unknown, the data were presented recalculated (see discussion in the Supplementary file). The original data, as far as it has been possible to reconstruct, are presented in Supplementary Table S9, with the caveat that quality assurance/control information is lacking and it was not possible to reconstruct the concentrations of the individual PCB congeners. The concentrations of various POPs are similar in ringed seals from northern Svalbard 1980 and ringed seals from Hornsund in 1984, whereas ringed seals from Kapp Linné seem to have higher concentrations; a similar pattern was observed for the bird samples in the Ymer-80 material.
Data on organochlorines in a ringed seal from Svalbard collected in 1980 is also presented by Andersson et al. (1988). However, as they used a different method of quantification, and presented only summary information of concentrations and samples, no relevant comparison of the results is possible. The cursory presentation of data by Andersson et al. (1988) is unfortunate; the only seal from Svalbard included in that paper is one of the Ymer-80 samples, and a detailed account of the results would have made a valuable comparison between methods possible.
Arctic char
Six different populations of Arctic char were sampled in Svalbard (Fig. 1), and one population on the east coast of Mainland, Shetland Islands. Summary data are given in Table 2, with details in Supplementary Table S5. The concentrations of toxaphene and individual cyclodiene pesticides were not possible to retrieve or reconstruct for individual fish, but the concentrations in pooled samples are given in Supplementary Table S6 for completeness.
In the anadromous populations of lakes Linnévatn, Diesetvatn and Arkvatn, some individuals reaching a certain size (or age) may spend a few summer weeks feeding in the sea or coastal lagoons (Hammar 1991). While the Arctic char collected from Diesetvatn were caught in a temporary, meromictic lagoon on Kapp Mitra, Kongsfjorden, away from their native freshwater system, the other individuals with an assumed partly marine feeding history had already returned to freshwater and amalgamated with the resident members of the populations. The Arctic char populations in the remaining lakes were landlocked, with such one-way obstructions in the outlets that return to their native freshwater, would be impossible.
Within some landlocked populations, such as in Annavatn and Wibjørnvatn, char of different size show very different feeding behaviour; small fish that feed mainly on zooplankton and insects grow slowly, while fish of above a certain size turn cannibalistic, which leads to faster growth, larger size, and accumulation of parasites (Hammar 2000). However, in these lakes with cannibalistic populations, during special events, for example, when chironomid or trichopteran pupae hatch, all sizes of Arctic char as well as both terrestrial and marine birds may feed on the insects. During these and other periods of the summer season, presence of bird droppings and remains of marine crustaceans and marine fish in char stomachs indicate a marine source of energy to landlocked Arctic char in the High Arctic (Skreslet 1973; Hammar 2000). A special case is Jensenvatn in which both small and large individuals foraged almost exclusively on the abundant amphipod Gammaracanthus lacustris (Hammar 2000). It is therefore not straightforward to compare the pollutant concentrations in these populations as their geography, ecology and life histories, including age, diet, growth and shifts of habitat, vary extensively both between and within the different populations and sampling sites (Hammar 1991, 2000). However, 31 individual PCB congeners and 10 pesticide compounds were determined in each sample, allowing a comparison of both contaminant concentrations and profiles with multivariate statistical methods.
A PCA of the relativized data shows clear differences between the lakes (Fig. 3). The similarity between Linnévatn in Svalbard and Girlsta Loch in Shetland is remarkable given the lakes are located in different archipelagos. Note, though, that economic activity in Svalbard is centred close to Linnévatn, and Girlsta Loch is located close to main economic centres in Shetland. The similarity between the two lakes implies that proximity to human activities was a dominant factor determining their contaminant profiles. Further, lakes Jensenvatn and Annavatn, situated close together further up the west coast, show similar overlapping convex hulls (i.e., appear close together in the PCA plot), while lakes Arkvatn and Wibjørnvatn, situated to the east along the north coast of Nordaustlandet (Fig. 1), separate out discretely from the other lakes. The two samples from Diesetvatn also separate out from the other lakes.
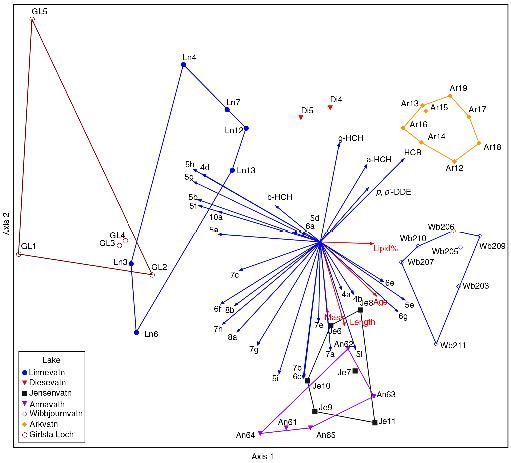
Fig. 3
Principal component analysis of relativized (see Supplementary file for details) contaminant concentrations in Arctic char with respect to lake. Axis 1 explains 50% and Axis 2 explains 22% of the total variation. Vector numbers refer to individual polychlorinated biphenyl (PCB) congeners; the numeral refers to the number of chlorines in the molecule, (see Supplementary Table S3b for full explanation). The samples from different lakes are presented as convex hulls. Lake Diesetvatn, with only two samples, is represented by the sample coordinates only. Identities of individual samples are given in Supplementary Table S5.
PCAs to evaluate the effect of sex and fish age did not detect any significant influence of these factors because of overlapping convex hulls (Supplementary Figs. S2, S3); that is, the pollutant pattern differences between the lakes were larger than the pollutant pattern differences between individuals in the same lake irrespective of age or sex. Interestingly, in both lakes from which we have samples of both smolt and resident parr, the parr had consistently lower total concentrations of all contaminants. Although the total concentration of contaminants increased with age, the pattern of the contaminants in relation to each other is unaltered.
Although there were some differences between anadromous and landlocked populations, they are not sufficient to separate these two (Supplementary Fig. S4). However, we have only had access to samples from two clearly anadromous individuals, both from Diesetvatn, and both of these fall outside of the area spanned by the landlocked lake systems in the PCA plot. It is possible that if more sea-run individual fish had been available, the differences between the systems would have been more obvious.
With respect to contaminant concentrations (Table 2, Supplementary Table S5), with the exception of the anadromous fish from Diesetvatn that showed the lowest concentrations of all contaminants, Arctic char from the three other lakes along the west coast of Svalbard (and also Girlsta Loch) have higher concentrations of PCB than the lakes on the north coast of eastern Svalbard. There is also a shift in the congener profiles in the lakes with fewer congeners found in Arkvatn and Wibjørnvatn, and with a shift towards more volatile congeners.
Among the western Svalbard lakes, char from lakes Jensenvatn and Annavatn in the north-west showed a different PCB congener profile as well as an overall higher concentration of ΣPCB than the char in Linnévatn (Table 2, Supplementary Table S5a), and the differences increase if only the resident individuals are compared. However, comparing with other contaminants, such as the presence of PCT, the similarities with Girlsta Loch char (Fig. 3), and the larger number of congeners in Linnévatn indicates proximity to a source of technical PCB. Given its location close to one of the major settlements and a telecommunications facility, it is logical that Linnévatn is more influenced by human than the other lakes. The concentration differences between the lakes, on the other hand, reflect differences in how PCB is transferred in their food chains.
The PCB concentrations in char from lakes Jensenvatn and Annavatn are striking. As pointed out above, the food chains in these two closely located lakes differ substantially. In spite of this, the contaminant concentrations and profiles in the lakes are similar (Fig. 2), indicating that the source of the contaminants is more important than the food chain for the contaminants in the Arctic char in these lakes. While ice-free, both lakes are visited by seabirds to wash and to feed on hatching insects (Hammar 2000), and the droppings of the birds may transport POPs from the marine to the lake ecosystems (Evenset et al. 2004). These lakes are also located close to the shore and may receive sea spray or seawater intrusion; Jensenvatn seems to have salty bottom water.
In contrast to these western lakes, the easternmost lakes are ice-covered for a longer period, are not visited by birds to the same extent and do not receive sea spray or seawater intrusion. However, to fully evaluate differences in the contamination situations in the lakes, renewed sampling is necessary. The determination of stable carbon and nitrogen isotope ratios (δ13C and δ15N) would also help in identifying sea-run individuals from resident ones and would also make comparisons of the trophic levels of the fish in the lakes possible.
Notably, the Arctic char from Linnévatn and Girlsta Loch had lower concentrations of HCB and the HCHs than the other lakes. The explanation could, perhaps, be that these two are ice-free for a longer time of the year than the other lakes, allowing these relatively volatile POPs to volatilize. It is also noteworthy that the Arctic char has generally higher concentrations of the HCHs than the birds and mammals. This may be due to species differences in metabolism, but also an effect of meltwater enrichment (Helm et al. 2002; Diamond et al. 2005), as meltwater can reach the lake ecosystems even if the lakes are covered by ice. Generally, in the early 1980s, HCHs were still being deposited in the Arctic (Li & Macdonald 2005), and the predicted concentrations for meltwater were higher than the predicted concentrations for seawater.
Comparison of data from 1971 and 1980
PCB and DDT data from three species, Brünnich’s guillemot, glaucous gull and polar bear, sampled in 1971 were summarized by Edelstam et al. (1987). These and adjusted Ymer-80 data are compared in Supplementary Table S8. The PCB and DDT concentrations from 1971 and 1980 appear to be within the same range for each of the three species. Further, the concentrations of the only polar bear sample from Ymer-80 that is comparable with other studies fall within the range of the concentrations reported for archived samples from 1967 (Derocher et al. 2003). It would, therefore, seem that the PCB and DDT concentrations in Svalbard were fairly similar in the late 1960s and early 1980s. As far as it is possible to draw any conclusions from these few data, they suggest that the PCB and DDT concentrations culminated in the Svalbard environment some time during the 1970s. This is similar to other studies; PCB and DDT concentrations in sediment from lake Ellasjøen seem to peak around 1970 (Evenset et al. 2007), and the deposition flux of PCB to the glacier Lomonosovfonna also shows a peak in the 1970s (Garmash et al. 2013), although more recent fluxes also seem to be high. Peaking PCB and DDT concentrations in the Arctic during the 1970s is consistent with increased environmental awareness and successive bans in western countries during this time.
Acknowledgements
The Polar Research Committee, Swedish Royal Academy of Sciences organized Ymer-80. The Royal Swedish Navy operated the ship. Bjørn Kjos-Hanssen collected the 1980 bird samples, and Magnar Norderhaug made available the 1971 samples. Johan Hammar financed the 1979 and 1981 Arctic char sampling privately. Erna and Victor Hasselblad Foundation funded sample analyses. We thank a reviewer for substantial and constructive comments.
References
AMAP (Arctic Monitoring and Assessment Programme) 2011. AMAP strategic framework 2010+. AMAP report 2010:8.
Oslo: Arctic Monitoring and Assessment Programme.
Andersson
Ö.,
Linder
C.E.,
Olsson
M.,
Reutergårdh
L.,
Uvemo
U.B.
&
Wideqvist
U. 1988.
Spatial differences and temporal trends of organochlorine compounds in biota from the northwestern hemisphere. Archives of Environmental Contamination and Toxicology 17,
755–765.
PubMed Abstract | Publisher Full Text
Carlberg
G.E.
&
Bøler
J.B. 1985. Determination of persistent chlorinated hydrocarbons and inorganic elements in samples from Svalbard. Report 83 11 01-1.
Oslo: Center for Industrial Research.
de Boer
J. 2000. Polychlorinated terphenyls. In
J.
Paasivirta (ed.): The handbook of environmental chemistry—new types of persistent halogenated compounds. Pp. 44–59.
Berlin: Springer.
de March
B.G.,
de Wit
C.A.
&
Muir
D.C. 1998. Persistent organic pollutants. In
S.J.
Wilson
et al. (eds.): AMAP assessment report: Arctic pollution issues. Pp. 183–372.
Oslo: Arctic Monitoring and Assessment Programme.
Derocher
A.E.,
Wolkers
H.,
Colburn
T.,
Schlabach
M.,
Larsen
T.S.
&
Wiig
Ø. 2003.
Contaminants in Svalbard polar bear samples archived since 1967 and possible population level effects. Science of the Total Environment 301,
163–174.
PubMed Abstract
| Publisher Full Text
Dewailly
E.,
Nantel
A.,
Weber
J.P.
&
Meyer
F. 1989.
High levels of PCBs in breast milk of Inuit women from Arctic Quebec. Bulletin of Environmental Contamination and Toxicology 43,
641–646.
PubMed Abstract | Publisher Full Text
Diamond
M.L.,
Bhavsar
S.P.,
Helm
P.A.,
Stern
G.A.
&
Alaee
M. 2005.
Fate of organochlorine contaminants in Arctic and Subarctic lakes estimated by mass balance modelling. Science of the Total Environment 342,
245–259.
PubMed Abstract | Publisher Full Text
Edelstam
C.,
Hammar
J.,
Jensen
S.,
Mowrer
J.
&
Olsson
M. 1987. Miljögifter i Polarhavet. Analysresultat från Ymer-expeditionen 1980. (Environmental pollutants in the Polar Sea. Results from the Ymer expedition 1980.)
In
G.
Hoppe
et al. (eds.): Expeditionen Ymer-80: en slutrapport. (Expedition Ymer-80: a final report.) Pp. 174–182.
Stockholm: Royal Academy of Sciences.
Evenset
A.,
Christensen
G.N.,
Carroll
J.,
Zaborska
A.,
Berger
U.,
Herzke
H.
&
Gregor
D. 2007.
Historical trend in persistent organic pollutants and metals recorded in sediment from lake Ellasjøen, Bjørnøya, Norwegian Arctic. Environmental Pollution 146,
196–205.
PubMed Abstract | Publisher Full Text
Evenset
A.,
Christensen
G.N.,
Skotvold
T.,
Fjeld
E.,
Schlabach
M.,
Wartena
E.
&
Gregor
D. 2004.
A comparison of organic contaminants in two High Arctic lake ecosystems, Bjørnøya (Bear Island), Norway. Science of the Total Environment 318,
125–141.
PubMed Abstract | Publisher Full Text
Garmash
O.,
Hermanson
M.H.,
Isaksson
E.,
Schwikowski
M.,
Divine
D.,
Teixeira
C.
&
Muir
D.C.G. 2013.
Deposition history of polychlorinated biphenyls to the Lomonosovfonna glacier, Svalbard: a 209 congener analysis. Environmental Science & Technology 47,
12064–12072.
PubMed Abstract | PubMed Central Full Text | Publisher Full Text
Hammar
J. 1991.
Speciation processes in the High Arctic: hardly as simple as the environment might suggest. International Society of Arctic Char Fanatics Information Series 5,
73–88.
Hammar
J. 2000.
Cannibals and parasites: conflicting regulators of bimodality in high latitude Arctic char, Salvelinus alpinus. Oikos 88,
33–47.
Publisher Full Text
Helm
P.A.,
Diamond
M.L.,
Semkin
R.,
Strachan
W.M.J.,
Teixeira
C.
&
Gregor
D. 2002.
A mass balance model describing multiyear fate of organochlorine compounds in a High Arctic lake. Environmental Science & Technology 36,
996–1003.
PubMed Abstract | PubMed Central Full Text | Publisher Full Text
Hoppe
G.,
Björn-Rasmussen
S.
&
Wiberg
R.M.
(eds.) 1987. Expeditionen Ymer-80: en slutrapport. (Expedition Ymer-80: a final report.)
Stockholm: Swedish Royal Academy of Sciences.
Li
Y.F.
&
Macdonald
R.W. 2005.
Sources and pathways of selected organochlorine pesticides to the Arctic and the effect of pathway divergence on HCH trends in biota: a review. Science of the Total Environment 342,
87–106.
PubMed Abstract | Publisher Full Text
Mehlum
F.
&
Daelemans
F.F. 1995.
PCBs in Arctic seabirds from the Svalbard region. Science of the Total Environment 160–161,
441–446.
Publisher Full Text
Muir
D.C.G.,
Norstrom
R.J.
&
Simon
M. 1988.
Organochlorine contaminants in Arctic marine food chains: accumulation of specific polychlorinated biphenyls and chlordane-related compounds. Environmental Science & Technology 22,
1071–1079.
PubMed Abstract | PubMed Central Full Text | Publisher Full Text
Norheim
G.
&
Kjos-Hanssen
B. 1984.
Persistent chlorinated hydrocarbons and mercury in birds caught off the west coast of Spitsbergen. Environmental Pollution Series A 33,
143–152.
Publisher Full Text
Norstrom
R.J.,
Simon
M.,
Muir
D.C.G.
&
Schweinsburg
R.E. 1988.
Organochlorine contaminants in Arctic marine food chains: identification, geographical distribution and temporal trends in polar bears. Environmental Science & Technology 22,
1063–1071.
PubMed Abstract | PubMed Central Full Text | Publisher Full Text
Sagerup
K.,
Savinov
V.,
Savinova
T.,
Kuklin
V.,
Muir
D.C.G.
&
Gabrielsen
G.W. 2009.
Persistent organic pollutants, heavy metals and parasites in the glaucous gull (Larus hyperboreus) on Spitsbergen. Environmental Pollution 157,
2282–2290.
PubMed Abstract | Publisher Full Text
Schytt
V. 1983.
Ymer-80: a Swedish expedition to the Arctic Ocean. Geographical Journal 149,
22–28.
Publisher Full Text
Skreslet
S. 1973.
The ecosystem of the Arctic lake Nordlaguna, Jan Mayen Island. III. Ecology of Arctic char, Salvelinus alpinus (L.). Astarte 6,
43–54.
Verreault
J.,
Gabrielsen
G.W.
&
Bustnes
J.O. 2010.
The Svalbard glaucous gull as bioindicator species in the European Arctic: insight from 35 years of contaminants research. Reviews of Environmental Contamination Toxicology 205,
77–116.
PubMed Abstract
Verreault
J.,
Muir
D.C.G.,
Norstrom
R.J.,
Stirling
I.,
Fisk
A.T.,
Gabrielsen
G.W.,
Derocher
A.E.,
Evans
T.J.,
Dietz
R.,
Sonne
C.,
Sandala
G.M.,
Gebbink
W.,
Riget
F.F.,
Born
E.W.,
Taylor
M.K.,
Nagy
J.
&
Letcher
R.J. 2005.
Chlorinated hydrocarbon contaminants and metabolites in polar bears (Ursus maritimus) from Alaska, Canada, East Greenland, and Svalbard: 1996–2002. Science of the Total Environment 351,
369–390.
PubMed Abstract | Publisher Full Text
Wania
F.
&
Mackay
D. 1996.
Tracking the distribution of persistent organic pollutants. Environmental Science & Technology 30,
390A–396A.
PubMed Abstract | PubMed Central Full Text | Publisher Full Text