RESEARCH/REVIEW ARTICLE
Hermit crabs (Pagurus spp.) at their northernmost range: distribution, abundance and shell use in the European Arctic
Piotr Balazy,1 Piotr Kuklinski,1,2 Maria Włodarska-Kowalczuk,1 David Barnes,3 Monika Kędra,1 Joanna Legeżyńska1
& Jan Marcin Węsławski1
1 Marine Ecology Department, Institute of Oceanology, Polish Academy of Sciences, Powstancow Warszawy 55, PL-81-712 Sopot, Poland
2 Department of Life Sciences, Natural History Museum, Cromwell Road, London SW7 5BD, UK
3 British Antarctic Survey, Madingley Road, High Cross, Cambridge CB3 OET, UK
Abstract
Hermit crabs are important components of Arctic benthic systems, yet baseline data on their densities and distribution patterns in this rapidly changing region are still scarce. Here we compile results of numerous research expeditions to Svalbard, the Barents Sea and northern Norway that were carried out from 1979 to 2011 by the Institute of Oceanology, Polish Academy of Sciences. The diversity of hermit crabs at the northern edge of their occurrence is very low; in Svalbard waters only one species (Pagurus pubescens) was detected. Another species (P. bernhardus), found in northern mainland Norway, north of the Arctic Circle, is likely to extend its distribution northward as the climate warms. Where the two species co-occur, competition between them probably accounts for the smaller sizes and poorer quality shells used by P. pubescens. The composition of the mollusc shells inhabited by these crabs differs between northern Norway and Svalbard, reflecting local mollusc species pools. Hermit crab densities were significantly higher than previously reported (max. mean 10 ind. m−2), suggesting their increasing level of dominance in benthic communities in the studied areas. The first to report the distribution of hermit crabs among habitats, this study showed that most individuals occurred at shallow depths (5–150 m), away from glacier termini and on hard bedrock rather than on soft substrata.
Keywords
Hermit crabs; Svalbard; Arctic; fjords.
Correspondence
Piotr Balazy, Marine Ecology Department, Institute of Oceanology, Polish Academy of Sciences, Powstancow Warszawy 55, PL-81-712 Sopot, Poland. E-mail: balazy@iopan.pl
(Published: 31 March 2015)
Polar Research 2015. © 2015 P. Balazy et al. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), permitting all non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Citation: Polar Research 2015, 34, 21412, http://dx.doi.org/10.3402/polar.v34.21412
Hermit crabs (Paguroidea, Decapoda) are common and abundant in almost all types of benthic habitats worldwide (Lancaster 1988). They are opportunistic feeders, scavengers or predators (Hazlett 1981) and are consumed by demersal fish, seabirds, brachyuran crabs, octopus and starfish (Lancaster 1988 and references therein). The wide distribution of hermit crabs is facilitated by their pelagic larvae: typically four free-swimming zoeal stages and one megalopal stage, which, after metamorphosis, turns into a young crab (Lancaster 1988). Due to a high fecundity—1200 eggs for a female Pagurus bernhardus—hermit crab larvae can be numerous in plankton communities (Jackson 1913; Thorson 1946; Bookhout 1964; Weydmann et al. 2013). In favourable laboratory conditions (10°C and salinity of 30–35), full development can be completed within 52–64 days (P. bernhardus, Bookhout 1964), but lower temperature and salinity along with reduced food availability or shortage of suitable habitat and resources (empty gastropod shells) may significantly prolong, or prevent, larval development (Bookhout 1964; Roberts 1971; Warner 1977; Dawirs 1979, 1981; Harms 1992; Harvey 1996). Through the use of gastropod shells pagurids provide transportation and secondary habitat for epibionts (Williams & McDermott 2004), typically harbouring higher numbers of associated species than other substrates, such as similar sized live gastropods or pebbles (Balazy & Kuklinski 2013). Therefore, they are a good example of ecosystem engineers (Stachowitsch 1977; McLean 1983; Jones et al. 1994, 1997; Reiss et al. 2003; Bell 2005; Balazy & Kuklinski 2013) and interesting model organisms for testing hypotheses on resource use and biodiversity patterns (Williams & McDermott 2004).
Previous research on hermit crabs has been mostly carried out in tropical and temperate seas (Abrams 1980; Barnes 1997, 1999; Barnes & De Grave 2000; Sandford 2003; Fransozo et al. 2007; Zuschin & Pille 2007; Ayres-Peres & Mantelatto 2008; Bach & Hazlett 2009; Tricarico et al. 2009). Studies from higher latitudes are still scarce, partly because of logistic constraints (but see Samuelsen 1970; Squires et al. 1993; Barnes et al. 2007; Kuklinski et al. 2008; Balazy & Kuklinski 2013; Peura et al. 2013). In Norwegian waters, pagurid fauna is represented by several species, including Anapagurus chiroacanthus, A. laevis, Pagurus alatus, P. bernhardus, P. cuanensis, P. prideaux and P. pubescens (Sandberg & McLaughlin 1998; Brattegard & Holthe 2001). The species richness of hermit crabs significantly declines northwards (Abele 1974), and is limited to three taxa (P. pubescens, P. bernhardus and A. chiroacanthus) in northern Norway (Sandberg & McLaughlin 1998; Barnes et al. 2007) and only two taxa in Svalbard waters. Pagurus pubescens, which has the most northward range among the North Atlantic hermit crabs, commonly occurs around the Svalbard Archipelago (Sandberg & McLaughlin 1998; Selbie 1921). The second species reported from this area, but rarely found, is P. bernhardus (Sandberg & McLaughlin 1998; Gulliksen et al. 1999; Gulliksen & Svensen 2004). This poleward impoverishment of hermit crab species is most likely the result of lowered temperatures (Lindley 1998) and is matched by an even more severe poleward decline in the Southern Hemisphere.
The ongoing environmental change (temperature increase, ice loss, acidification) and increase of anthropogenic pressure (oil drilling and fisheries) may lead to substantial ecological changes within Arctic benthic communities (Core Writing Team et al. 2007; Dawson et al. 2011; Wassmann et al. 2011; Węsławski et al. 2011; Duarte et al. 2012; Wang & Overland 2012). Climate-forced northward expansions have been observed for boreal species (Berge et al. 2005) and predicted for cold-water Arctic species (Węsławski et al. 2010; Węsławski et al. 2011). In Svalbard, hermit crabs of the genus Pagurus occur at their northernmost limit (Birula 1907; Heegaard 1941) and may therefore act as good indicators of physical changes. Increased levels of oceanographic variability (Walczowski & Piechura 2006; Walczowski et al. 2012) and fishery-related disturbance (Berge et al. 2009) will likely have a positive effect on their distribution range, species richness and abundance. Berge et al. (2009) have recently proved that the composition of decapod fauna in Spitsbergen waters has not changed during the last 50 years. On the other hand, they have found evidence for changes in the decapod community structure, with opportunistic scavenging crabs becoming increasingly dominant and specialized predatory shrimps correspondingly decreasing.
Knowledge of pagurid distribution, density and gastropod shell use remains scattered and incomplete in the Arctic. To the best of our knowledge the only study available from Svalbard waters is by Barnes et al. (2007); however, the scale of this was limited and it did not cover substratum choice. Here we compile data gathered during numerous research expeditions carried out by the Institute of Oceanology of the Polish Academy of Sciences (IO PAN) during the last four decades to Svalbard, the Barents Sea and northern Norway. Samples were taken across a range of depths (3–3000 m) and habitats in an attempt to establish robust baseline information as a reference or starting point for further ecological assessments.
Material and methods
Study area
This study was carried out in northern Norway (69°N) and two High-Arctic areas: the Barents Sea's shallow bank (Svalbard Bank) and the western coast of Spitsbergen, the biggest island of the Svalbard Archipelago (Fig. 1). The hydrology of these areas is shaped by the interplay of the two large water masses of Atlantic and Arctic Ocean origin. Three sites selected around Tromsø in northern Norway (Kvalsund, Mjøsund, Grøtfjord) as well as the western side of Spitsbergen are influenced by the relatively warm and saline (T>3°C, S > 35; Loeng 1991) waters of the North Atlantic and the West Spitsbergen Current. Despite their high latitude (76–80°N) the west Spitsbergen fjords have a mild, rather than Arctic character (Hop et al. 2002; Svendsen et al. 2002). They remain ice-free most of the year; during winter the surface water freezes for a few months (Węsławski et al. 1988). The southernmost fjord of the island, Hornsund, is strongly affected by slightly less saline and colder (T<0°C, S 34.3–34.8; Loeng 1991) waters originating in the Arctic Ocean and transported by the East Spitsbergen Current (Swerpel 1985). A front between North Atlantic and Arctic water masses passes both sides of the Svalbard Bank, an elongated shallow bank that rises from the bottom of the Barents Sea up to 30 m under the surface. This largest open-shelf cold-water carbonate platform in the Arctic, built from shell fragments mixed with very coarse sand and gravel (Elverhøi & Solheim 1983; Henrich et al. 1997) is one of the most productive areas in the Barents Sea (Sakshaug et al. 2009). In its central part, Arctic water mixes with the meltwater which has been heated by the atmosphere during the summer (T 1–3°C, S<34.4; Loeng 1991). Freshwater runoff from melting glaciers or glacier-fed rivers has a significant impact on the hydrological conditions prevailing inside the fjords. Apart from decreased salinity and iceberg scouring, large amounts of sedimenting mineral particles can result in declines in benthic species richness, diversity and abundance, especially in the innermost parts of the fjords, close to tidal glaciers (Włodarska-Kowalczuk et al. 2005). The sediment accumulation rates in Kongsfjorden, the fjord with the most active glacier in the Svalbard Archipelago—Kongsbreen (Lefauconnier et al. 1994), range from 20 000 g m−2 year−1 near the glacier front to 200 g m−2 year−1 at the fjord mouth (Svendsen et al. 2002). As a result, the fjords seafloor is mainly covered by soft homogeneous mud with ice-rafted clasts (Elverhøi et al. 1983). Hard substrata habitats—rocky shelves and mixed boulder fields with coarser fractions such as pebbles, cobbles and boulders—mostly occur at the outer parts of the fjords, where stronger bottom currents occur (Kaczmarek et al. 2005). Soft bottom macrobenthic assemblages are dominated mostly by infaunal, deposit feeding or carnivorous bivalve and polychaete species (Włodarska-Kowalczuk et al. 1998; Włodarska-Kowalczuk et al. 2007). In the shallow subtidal zone of outer fjord basins, dense algae (Laminaria spp.) and associated diverse epibenthic assemblages of ascidians, barnacles, bryozoans, cnidarians, sedentary polychaetes and sponges occur on hard bedrock shelves (Barnes & Kuklinski 2005; Barnes et al. 2007). Hermit crabs (Pagurus spp.), other decapods, such as the spider crab Hyas araneus or shrimps (Lebbeus polaris, Eualus gaimardii, Sclerocrangon boreas) are common benthic predatory and scavenging fauna in these habitats (Kaczmarek et al. 2005; Berge et al. 2009).
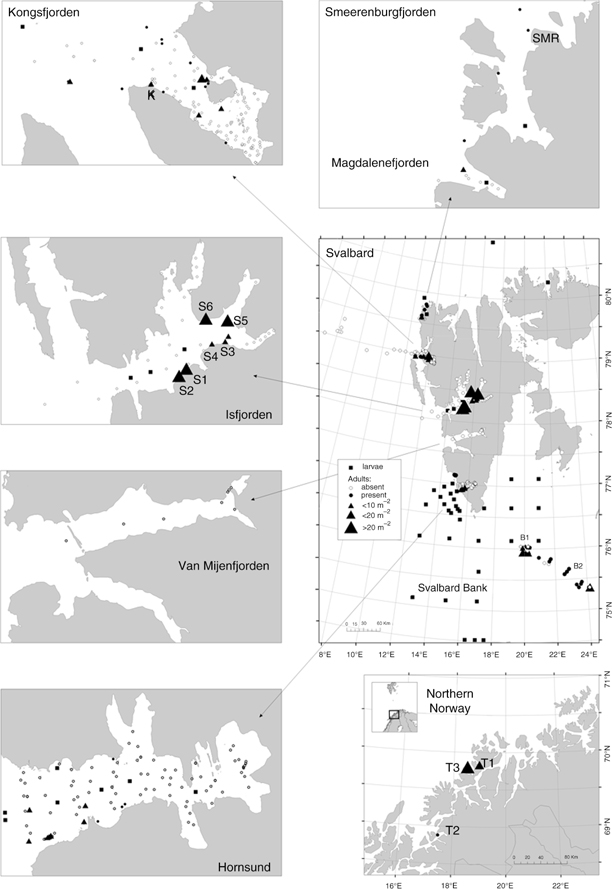
Fig. 1
Distribution and abundance of hermit crabs Pagurus spp. in the studied areas. Data on pelagic zoea larvae are from Węsławski et al. (1991), Węsławski, Koszteyn et al. (1999), Węsławski, Stempniewicz et al. (1999), Kwasniewski et al. (2010), Jakubas et al. (2011), Kwasniewski et al. (2012), Weydman et al. (2013) and Gluchowska et al. (unpubl. ms.).
Sampling
Material was collected during cruises of the RV Oceania and land-based expeditions to the Svalbard Archipelago organized by IO PAN in the summer seasons between 1979 and 2011. Material was collected at the Svalbard Bank by the RV Oceania in 2009. Samples in northern Norway, were collected during land-based expeditions in 2009 and 2010. In total, 240 stations were sampled from the major fjords of west Spitsbergen, at Svalbard Bank and at three sites in northern Norway, across a depth range of 2–2977 m. Most of the sampling sites were localized in two west Spitsbergen fjords—Hornsund and Kongsfjorden (Fig. 1)—both European Marine Biodiversity Research Sites (Warwick et al. 2003).
Samples were collected using different sampling methods that were considered most appropriate across depth zones and habitats (Table 1). Quantitative samples from the deeper subtidal (>30 m) were collected using a 0.1 m2 van Veen grab or 0.045 m2 Petit Ponar grab. Presence/absence data were scored from dredge sampling as well as videos recorded by a benthic lander and an underwater “bottom-looking” drop camera. In shallow (<20 m) areas unsuitable for sampling directly from research vessels, and on bottom types where conventional surface-operated sampling gear has a limited efficiency (e.g., hard bedrock), samples were collected by SCUBA divers. In this case, hermit crab density estimates were obtained with the use of a (0.5×0.5 m) 0.25 m2 quadrat. We counted all individuals within each of three quadrats placed haphazardly on the sea bottom at 3, 5, 10, 15 and 20 m depths. At each site, the bottom type was classified by visual inspection by divers as hard bedrock (sites S1, S2, S5), mixed substrata (sand and gravel; sites S6, T1, T3) or soft bottom (sand and mud; sites S3, S4). The samples were fixed in 4% buffered formaldehyde and transported to the laboratory.
Table 1 Details of sampling campaigns. Abundance values are mean (±standard error) and, in italics, maximum abundance.
| Year |
Site |
Depths (m) |
Sampling technique |
Number of stations |
Number of samples |
Abundance
(ind. m−2) |
Reference |
| 2009 |
Smeerenburgfjorden |
7–177 |
SCUBA diving
dredge |
4 |
4 |
Only qualitative |
This paper |
2007
2009 |
Magdalenefjorden |
12–103 |
SCUBA diving
dredge
Van Veen grab |
7 |
17 |
10 (±0.00) 10
|
This paper |
1996–2001
2006
2009 |
Kongsfjorden |
4–2977 |
SCUBA diving
dredge
Van Veen grab |
102 |
239 |
6 (±0.45) 20
|
Włodarska-Kowalczuk et al. 2004
Kaczmarek et al. 2005
Kędra et al. 2010 |
| 2009–2011 |
Isfjorden |
3–289 |
SCUBA diving
dredge
Van Veen grab |
35 |
119 |
7 (±0.11) 44
|
Balazy & Kuklinski 2013
This paper |
| 2000–2001 |
Van Mijenfjorden |
4–104 |
Van Veen grab
Petit Ponar grab |
8 |
24 |
Absent |
This paper |
1979–1982
2002–2003
2005
2007
2009 |
Hornsund |
11–237 |
SCUBA diving
dredge
Van Veen grab
Benthic lander |
84 |
138 |
5 (±0.31) 10
|
This paper |
| 2009 |
Svalbard Bank |
39–150 |
Dredge
Van Veen grab
Benthic lander |
17 |
70 |
4 (±0.24) 30
|
Kędra et al. 2013 |
| 2009–2011 |
Northern Norway |
3–20 |
SCUBA diving |
3 |
15 |
7 (±0.30) 28
|
This paper |
Hermit crab species, gender, shield length, wet mass, presence of parasites and overgrowing epibionts (larger >1 mm) were noted. Crabs were classified into five size classes on the basis of shield length (class 1: 0–3.5 mm; class 2: 3.5–5.0; class 3: 5.0–6.5; class 4: 6.5–8.0; class 5: above 8.0). Gastropod shells were identified to species or, when damaged, to the lowest possible taxonomic level.
Statistical analysis
Due to significant heterogeneity of variances, a non-parametric Kruskal–Wallis test was used to test for differences in hermit crab abundance with depth and size. Post-hoc pair-wise testing was performed with the use of Mann-Whitney's U-test. The Hurlbert rarefaction diversity index (ES[n]) (Hurlbert 1971) was used in order to compare diversity of gastropod shells used by hermit crabs between the sites with different numbers of individuals collected. Accumulation curves of the different gastropod shell types used were plotted for samples collected at each site. Species accumulation curves, ES[n] were computed and plotted with the PRIMER version 6 software package (Clarke & Gorley 2001). The statistical analyses were performed in STATISTICA version 10 (StatSoft Inc.).
Results
Distribution and abundance
Hermit crabs were present in all study areas except for Van Mijenfjorden, a relatively poorly sampled fjord in Spitsbergen (Fig. 1). The most northerly site with hermit crabs present was Smeerenburgfjorden (79.8°N). Hermit crabs were most abundant in Isfjorden, where abundance reached 44 ind. m−2 in a single sample (Table 1, Fig. 1). Up to 30 ind. m−2 were found at Svalbard Bank, while in northern Norway maximum abundance was 28 ind. m−2. Mean abundances calculated from all samples collected at a given site never exceeded 10 ind. m−2 (Table 1).
Very few crabs were found in the innermost part of the study fjords and none close to glaciers. Hermit crabs in Spitsbergen fjords were found only between 5 m and 150 m depth despite samples being collected at greater depths (Fig. 2). At Svalbard Bank, they similarly occurred down to 140 m.
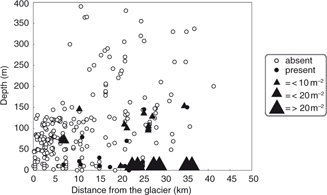
Fig. 2
Hermit crab occurrence and abundance in relation to depth and distance to glaciers.
In the shallow sublittoral (3–20 m), diver-held quadrats revealed significant differences in abundance of hermit crabs among different depth zones (Kruskal-Wallis, H(4, 114)=20.78, p<0.001; Fig. 3): shallow (3 and 5 m) versus deeper (10, 15 and 20 m) (Mann-Whitney U, p<0.05). The mean abundance was significantly higher at 9–10.7 ind.m−2 at 10–20 m compared to 1.7–2.7 ind.m−2 at 3–5 m. This “overall” abundance–depth pattern was driven by significant depth differences at bedrock sites (Kruskal–Wallis, H(4,45)=28.03, p<0.001; Mann-Whitney U, p<0.01; Fig. 3). However, on mixed and soft bottom sites we found no differences with depth (Kruskal–Wallis, p>0.05). The differences between the three bottom types were found at each study depth (Kruskal–Wallis, p<0.05; Fig. 3) except 10 m (Kruskal–Wallis, H(2,24)=5.83 p=0.05). At 15 and 20 m, hermit crabs were more abundant at sites with hard (mean 16.4 and 16.7 ind. m−2, respectively) than soft substrata (mean 2.0 and 1.3 ind. m−2; Mann-Whitney U, p<0.05). Samples from mixed substrata in this depth range did not differ significantly from the other two bottom types (mean 10.0 and 9.3 ind. m−2; Mann-Whitney U, p>0.05). No hermit crabs were found at the shallowest depths at sites with soft (3 m) and hard bedrock (3 and 5 m). Mean hermit crab abundance (7.1 at 3 m and 0.7 ind. m−2 at 5 m) on mixed substrata differed significantly from those at 3 and 5 m on bedrock (Mann-Whitney U, p<0.05) and 3 m on soft substrata (Mann-Whitney U, p<0.05).
Species, size, gender and parasitism
Pagurus pubescens was the only species found at Svalbard Bank and in Spitsbergen fjords, whilst in northern Norway P. bernhardus was also noted and comprised 34% of all individuals collected. Each species in northern Norway had different size ranges, as measured in shield lengths: P. pubescens 2.08–7.80 mm; P. bernhardus 1.40–10.46 mm. Specimens of P. bernhardus (mean 6.7 mm) were significantly larger than P. pubescens (mean 4.7, Mann-Whitney U, p<0.001). Males of both hermit crab species were significantly larger in shield length than females (Mann-Whitney U, p<0.05). Gender ratios were close to equal.
Across the study areas 17% of P. pubescens were parasitized by Peltogaster paguri but just 6% of both species were overgrown by epibionts (juvenile serpulids, spirorbid Circeis armoricana, cirriped Semibalanus balanoides, bryozoan Patinella sp. and hydroids). Significant differences in the size of hermit crabs were found between sites (shield length: Kruskal-Wallis, H(9,391)=65.79, p<0.001; wet mass: Kruskal–Wallis, H(9,391)=78.23, p<0.001; Fig. 4). In terms of shield length and wet mass, the largest specimens were P. bernhardus collected at site T3 in northern Norway (mean shield length 8.2 mm and wet mass of 4.0 g). Specimens collected at this site were nearly two times longer and over four times heavier than those from (nearby) site T2, northern Norway. The largest P. pubescens were found at the S2 site in Isfjorden (mean shield length 6.9 mm and wet mass of 2.6 g).
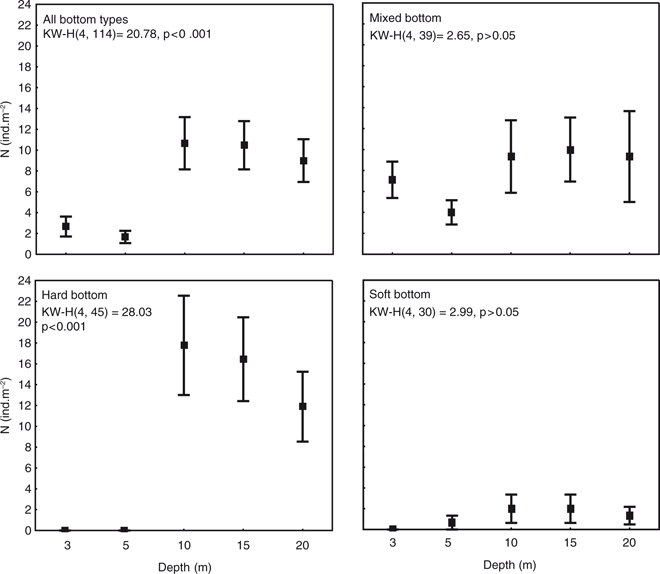
Fig. 3
Mean values with standard errors of hermit crab (Pagurus spp.) abundance at different depths and bottom types. Only samples collected in shallow subtidal (depths down to the 20 m) are presented.
Shell use
Hermit crabs inhabited shells belonging to 23 taxa (Table 2). Species accumulation curves showed that the highest richness of gastropod shells (11 taxa) were used by P. pubescens at Svalbard Bank site B2 and Smeerenburgfjorden, Spitsbergen (Fig. 5). Pagurus bernhardus was found in the fewest types of shells (in northern Norway, three at T1 and T2; four at T3). However, this species was also less numerous in samples taken (Table 2, Fig. 5). Interpolation of gastropod richness to the same level of 15 samples among all sites (as only 15 individuals of P. bernhardus were collected at T1) suggested that P. bernhardus inhabited fewer gastropod shell types than P. pubescens. (In northern Norway, at site T1 there were three types for P. bernhardus and five for P. pubescens; at site T2, three for P. bernhardus and seven for P. pubescens. At site T3, both P. bernhardus and P. pubescens had four.) However, an ES[15] analysis and species accumulation plot did not reveal any clear differences in the use of gastropod shells by P. pubescens with latitude. (In northern Norway, at site T1 there were five, at T2 seven and at T3 four; at the Svalbard Bank, at site B1 there were four and at B2 seven; in Spitsbergen, at site S1 there were seven, at S2 four, at S3 six, at Kongsfjorden five and at Smeerenburgfjorden six.)
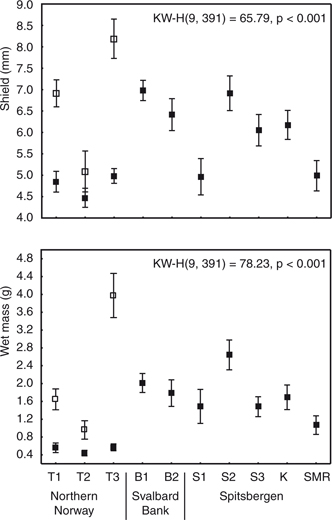
Fig. 4
Shield length and wet mass of hermit crabs (open squares, Pagurus bernhardus; filled squares, P. pubescens) collected at sites in northern Norway (T1, T2, T3), the Svalbard Bank (B1, B2) and Spitsbergen (S1, S2, S3, S4, K, SMR). Mean values with standard errors and results of a Kruskal-Wallis test for difference among sites are presented.
Table 2 Shell use by Pagurus pubescens (Pp), P. bernhardus (Pb) at sites in northern Norway, at the Svalbard Bank and on Spitsbergen. Pp and Pb in boldface denotes more than five individuals; normal type represents five or fewer individuals.
| |
Northern Norwaya
|
Svalbard Bankb
|
Spitsbergenc
|
| Shell species |
T1 |
T2 |
T3 |
B1 |
B2 |
S1 |
S2 |
S3 |
K |
SMR |
| Amauropsis islandica
|
|
Pp |
|
|
|
|
|
|
|
|
| Boreotrophon truncatus
|
|
|
|
Pp |
|
Pp |
|
Pp |
|
Pp |
| Buccinum glaciale
|
|
|
|
Pp |
Pp
|
Pp
|
Pp
|
Pp
|
Pp |
Pp
|
| Buccinum polare
|
|
|
Pb |
|
|
|
|
Pp |
Pp |
Pp |
| Buccinum scalariforme
|
|
|
Pp Pb |
|
Pp |
Pp |
|
|
Pp
|
Pp |
| Buccinum undatum
|
Pp Pb |
Pp Pb |
Pp Pb
|
Pp
|
Pp |
Pp |
Pp
|
Pp
|
Pp
|
Pp
|
| Buccinum sp. |
|
|
|
Pp
|
|
Pp
|
Pp |
Pp |
|
Pp |
| Colus kroeyeri
|
|
|
|
|
Pp |
Pp |
Pp |
|
|
Pp |
| Colus latericeus
|
|
|
|
|
|
Pp |
|
|
|
|
| Buccinidae
|
|
|
Pp |
|
Pp |
|
|
|
Pp |
Pp |
| Cryptonatica affinis
|
Pb |
|
|
Pp
|
Pp
|
|
|
Pp
|
Pp |
Pp |
| Lunatia montagui
|
|
Pp |
|
|
|
|
|
|
|
|
| Lunatia pallida
|
|
|
|
Pp |
Pp |
|
|
Pp
|
Pp |
Pp |
| Naticidae
|
|
|
|
Pp |
Pp |
|
|
|
|
|
| Gibbula cineraria
|
Pp |
Pp Pb |
Pp |
|
|
|
|
|
|
|
| Littorina littorea
|
Pp Pb
|
Pp Pb
|
Pp Pb |
|
|
|
|
|
|
|
| Littorina obtusata
|
Pp |
Pp |
|
|
|
|
|
|
|
|
| Margarites groenlandicus
|
|
Pp |
|
|
Pp |
Pp
|
Pp
|
|
Pp |
Pp
|
| Nucella lapillus
|
Pp |
Pp |
Pp
|
|
|
|
|
|
|
|
| Oenopota pyramidalis
|
|
|
|
|
|
Pp |
|
Pp |
|
|
| Muricidae |
|
|
|
|
Pp |
|
|
|
|
|
| Neogastropoda |
|
|
|
|
Pp |
|
|
|
|
|
| Undetermined |
|
Pp |
|
|
|
Pp |
Pp |
Pp |
|
|
| aAt site T1, there were 15 P. bernhardus individuals and 19 P. pubescens; at T2, there were 19 P. bernhardus and 25 P. pubescens; at T3, there were 18 P. bernhardus and 32 P. pubescens. bAt the Svalbard Bank there were 50 individuals at each site. cAt each site in Spitsbergen (Isfjorden: S1, S2, S3; Kongsfjorden: K; Smeerenburgfjorden: SMR), there were 50 individuals. |
Shells of Buccinum undatum were used at all study sites and by both hermit crab species. Shells belonging to other members of the Buccinidae family (B. glaciale, B. scalariforme and Colus kroeyeri) were also common in collected material (Table 2). In northern Norway, 42% of all shells used by P. bernhardus belonged to the common periwinkle Littorina littorea and 38% came from the large whelk B. undatum. The other hermit crab species, P. pubescens, used mostly Nucella lapillus (34%), Littorina littorea (17%) and L. obtusata (13%) in northern Norway. In contrast, at Svalbard Bank and Spitsbergen, the most inhabited shells were B. undatum (26%), B. glaciale (23%) and Margarites groenlandicus (14%). In general, the majority of small P. bernhardus used Gibbula cineraria shells. Medium-sized individuals inhabited mainly L. littorea shells, whilst the largest were found primarily in shells of Buccinum spp. (B. undatum, B. scalariforme and B. polare; Fig. 6). The species composition of shells used by P. pubescens found in northern Norway was similar, (except one additional gastropod species: Nucella lapillus), but the proportion in which they were used differed across size classes. The smallest P. pubescens individuals occupied G. cineraria, L. obtusata and L. littorea shells. In medium size classes, N. lapillus constituted a significant part, while the largest grouping (class 4) was dominated by Littorina spp. At Svalbard Bank and Spitsbergen, Buccinum spp. predominated in almost all size classes. The smallest P. pubescens in those two areas inhabited mainly shells of M. groenlandicus and Cryptonatica affinis, which was more plentiful at Svalbard Bank (Fig. 6).
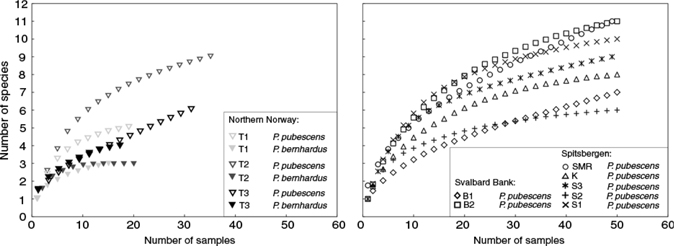
Fig. 5
Species accumulation curves of gastropod shells types used by hermit crabs.
In northern Norway, 31% of shells used by P. bernhardus and 34% of shells used by P. pubescens were defective shells with cracks and holes. Damaged shell use was higher in Spitsbergen (41%) than at Svalbard Bank, where damaged shells accounted for only 12%. No universal trend in the use of broken shells was observed in the five hermit crab size classes among the study areas (Fig. 7). In P. bernhardus, the highest proportion of damaged shells were used by small individuals, however, these data should be treated with caution as the sample size was small. The largest size classes of P. pubescens in northern Norway and Svalbard Bank used unbroken shells, but again, the northern Norway data set had few samples. At Spitsbergen, the largest proportion of broken shells was recorded in the medium (3) and large size classes (4, 5). Unsurprisingly, there was a strong positive correlation between hermit crab wet mass and mass of its shell (r2=0.787, t=26.09, p<0.001).
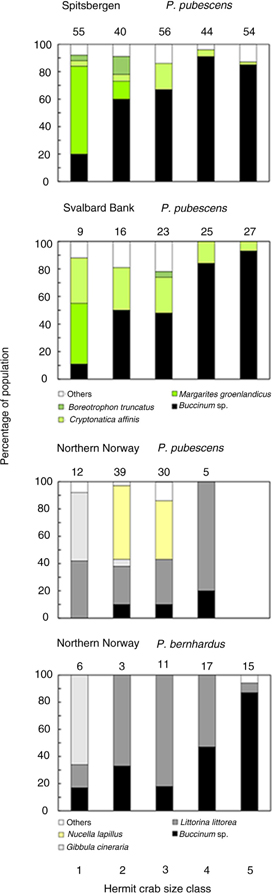
Fig. 6
Percentage of shell used by five hermit crab size classes in different areas. Above each column number of collected individuals is presented.
Discussion
Hermit crab diversity, distribution and abundance
Despite the fact that the current study attempted to cover large geographic areas we found only two species (P. bernhardus and P. pubescens) from just one genus in northern Norway and only the latter species at Svalbard Bank and in Spitsbergen. The Svalbard Archipelago is known as the northernmost (and lower temperature) limit of hermit crab occurrence (Birula 1907; Heegaard 1941) so a paucity of species is unsurprising. A previous study by Barnes et al. (2007), performed across similar sites, reported a single specimen of another hermit crab species, Anapagurus chiroacanthus, in northern Norway (at T2, Fig. 1). For Svalbard waters, the regional species checklists also report the presence of P. bernhardus (Sandberg & McLaughlin 1998; Gulliksen et al. 1999; Gulliksen & Svensen 2004; Palerud et al. 2004). However, it appears to be very rare here as among 630 specimens collected during a five-year study in Isfjorden, Spitsbergen (PB & PK, unpubl. data), and among 347 hermit crab specimens gathered in other fjords of Spitsbergen (Barnes et al. 2007), no representative of P. bernhardus was found. Furthermore, it was not found in other studies that focused on the decapod fauna in Svalbard waters (Christiansen & Christiansen 1962; Węsławski 1987; Duris 1995; Berge et al. 2009). Therefore, we assume that the established range of this species’ geographical range does not extend this far north. Sokolov (2006) classifies P. bernhardus as a warm Atlantic or Atlantic–Mediterranean species with a range that follows the branches of the Norwegian Current along the western coasts of Norway. Pagurus pubescens is defined as a eurythermic and euryhaline decapod that is widely distributed in eastern Arctic seas. If the trend of significant regional warming continues in the Arctic the range of P. bernhardus would seem likely to extend northwards and be reported more commonly in the Svalbard Archipelago. Such climate-forced range expansions are arguably already being observed, e.g., the recent reappearance of the mussel Mytlius edulis in west Spitsbergen fjords (Berge et al. 2005).
The only fjord surveyed in this study where no hermit crabs were found was Van Mijenfjorden. This is a semi-closed fjord system with some anthropogenic impact as there is an active coal mine located nearby. However, significantly measurable effects of mining activities on marine fauna have been described as restricted to places where the ships are loaded (Renaud et al. 2007). It is therefore most likely that the absence of hermit crabs in our Van Mijenfjorden material results from low sampling effort, as only eight stations were investigated.
No clear, large-scale trend in hermit crab abundance was apparent along the geographical gradient we studied (from northern Norway to Svalbard). Instead, abundance turned out to be highly variable between sites, suggesting that local factors are decisive. Interestingly, the abundance figures in the current study found abundance were higher than those reported by Barnes et al. (2007) at the same sites in 2005 (7 versus 2 ind. m−2 in northern Norway, 7 versus 2 ind. m−2 in Isfjord and 6 versus 0.8 in Kongsfjorden, this study). Berge et al. (2009) speculated that a shift from specialist predators towards opportunistic, scavenging species, like hermit crabs, might have occurred over the last 100 years in Isfjorden. Our results support this hypothesis; however, due to paucity of the time series, annual fluctuations driven by natural factors such as variability in hydrological regimes and seawater temperature cannot be ruled out (Renaud et al. 2008; Berge et al. 2009). Just after Barnes et al. (2007) undertook their sampling, in 2005, a huge northward advance of Atlantic waters and the highest recorded temperatures of the Spitsbergen Current were noted (Walczowski et al. 2012). Such a hydrologic event could increase the influx of larvae from lower latitudes and accelerate their development and enhance their survival rate (Bookhout 1964; Dawirs 1979; Lindley 1998).
As hermit crabs are mobile and capable of rapid dispersal (by larvae), their absence in fjord head regions suggests that they prefer conditions and/or habitats located in central and outer basins, away from sources of glacial or glaciofluvial outflows (usually in inner fjords). Glaciers and glacier-fed rivers transport large amounts of freshwater and mineral material into the sea and strongly impact neighbouring benthic systems. Sediments there are frequently resuspended and redeposited by gravity flows (Zajączkowski & Włodarska-Kowalczuk 2007), subjecting benthic communities to physical disturbance by heavy sedimentation of mineral particles. These communities have impoverished standing stocks and diversity compared to outer fjord regions (Włodarska-Kowalczuk et al. 2007). Larger, slow-moving organisms, such as hermit crabs, can be locally eliminated by unstable substrates that are often covered with a “fluffy layer” of unconsolidated sediment (Włodarska-Kowalczuk et al. 2005). High turbidity in surface waters together with strong mineral particles deposition result in low organic carbon concentrations in sediments of inner basins (e.g., Winkelmann & Knies 2005). This can have an additional negative effect on hermit crab populations as organic matter may constitute a supplementary source of food for deposit-feeding hermit crabs (Greenwood 1972; Gerlach et al. 1976; Fransozo et al. 1998).
A wide depth range has been reported for P. pubescens (5–1079 m; Ingle 1993) and P. bernhardus (intertidal: 1800 m; Sandberg & McLaughlin 1998). However, our data suggest that in the Arctic and sub-Arctic fjords and coastal waters they occur mostly at depths shallower than 150 m (Fig. 2). Similarly, around the British Isles, P. pubescens and P. bernhardus normally occur from 100 to 140 m, and are only occasionally found as deep as 500 m (Hayward & Ryland 1999). In Isfjorden, P. pubescens has its maximum abundance above 50 m (Christiansen & Christiansen 1962). In Hornsund and adjacent waters of southern Spitsbergen, the highest concentrations of P. pubescens were noted between 20 and 30 m (Magdziarz 1984) and between 21 and 50 m (Węsławski 1987), which is consistent with our results (see Fig. 2). A scarcity of Pagurus spp. in the shallow waters (3–5 m) is most probably the result of high physical disturbance (wave and ice action) in the Arctic. We did not observe any hermit crab ashore at low tide, whereas in lower latitudes (e.g., in the British Isles) they are widely recorded in rocky tide pools and the intertidal zone (Hayward & Ryland 1999).
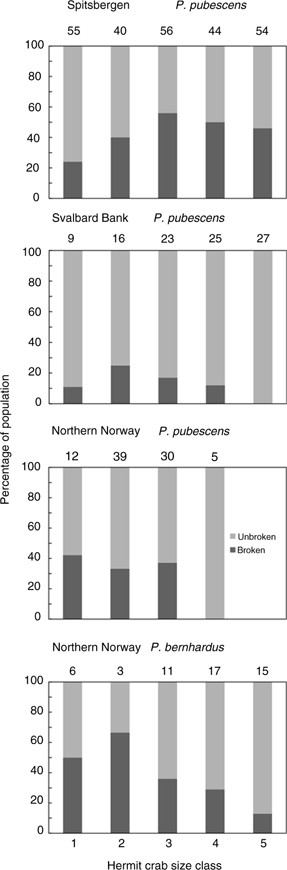
Fig. 7
Percentage of broken shell used by five hermit crab size classes in different areas. Above each column number of collected individuals is presented.
Substrate type is typically an important factor that determines the distribution of many benthic species, and this includes hermit crabs (Samuelsen 1970; Lancaster 1988 and references therein). As reported here, Magdziarz (1984) found that P. pubescens at Hornsund occurred mostly on hard (55%) or mixed bottom (rocks and mud, 37%), and only 8% on soft substrata. Węsławski (1987) also noted that, except for mud and the phytal zone, hermit crabs were mostly found associated with stones and coralline red algae. Likewise Eriksson et al. (1975) showed that P. bernhardus, prefers hard substratum to sand. Preference for hard substrata may actually be more prevalent than data suggest, given that the crevices that often characterize hard substrata make counts likely to be under-representations whilst on soft substrata the crabs are particularly conspicuous and difficult to overlook. More complex habitats support higher megafaunal densities (Robinson & Tully 2000) as they are usually more heterogeneous in structure and offer more food supplies and/or better shelter from predators (Wahle & Steneck 1991; Stelle 1999; Linnane et al. 2002; Moksens 2002; Pallas et al. 2006). Even with the protection of gastropod shells, hermit crabs can be easily detected on monotonous mud or sand surfaces and predated by demersal fish (e.g., wolfish [Anarhichas lupus] or ballan wrasse [Labrus bergylta]; Samuelsen 1970; Falk-Petersen et al. 2010), mammals (bearded seal [Erignathus barbatus], grey seal [Halichoerus grypus], walrus [Odobenus rosmarus]; Fay & Burns 1988; Lydersen et al. 1989; NAMMCO 1997), larger hermit crabs (PB, pers. obs.) or other bottom-dwelling predators (Lancaster 1988 and references therein).
Hermit crab size
The maximum size of hermit crabs is species- and genus-specific. Pagurus bernhardus is generally larger than P. pubescens (Sandberg & McLaughlin 1998; Hayward & Ryland 1999). Previous findings from other regions suggest that in areas of sympatry with an aggressively dominant species, smaller hermit crab species may be forced to inhabit smaller shells (than normal) and therefore reach typically smaller sizes than in nearby, monospecific areas (Bach et al. 1976; Hazlett 1981 and references therein). The less competitive individuals forced to occupy lower quality shells have also been shown to have a worse reproductive success (e.g., Elwood et al. 1995). It is unknown whether this is the case in northern Norway, where the two species of hermit crabs co-occur. We found that P. pubescens from Spitsbergen was, in general, larger in size than P. pubescens from northern Norway. It has been suggested that some arthropods in cold waters can grow to larger sizes as a result of higher oxygen availability and have increased life spans at lower temperatures (Chapelle & Peck 1999, 2004). This factor might be also responsible for differences in sizes observed between warmer northern Norway and colder, higher latitude sites. At a local scale, many other factors, like variable food supplies, shell resources, salinity or predation, can influence hermit crab sizes (Kellogg 1976; Bertness 1981a; Yoshino et al. 2002; Davenport 1972). In this study, sites varied considerably in terms of environmental conditions, making it hard to pinpoint factor(s) with the highest impact on hermit crab size. The smallest individuals were mainly found closer to river mouths (e.g., S1), highly influenced by freshwater runoff. Many of the largest individuals occurred offshore (Svalbard Bank), close to the frontal zone of very productive and nutrient-rich water masses.
Shell use
Results from the present study indicate that, except for one site (T3), P. bernhardus used fewer shell types than P. pubescens. Both species have been reported earlier to utilize shells of a similar number of gastropod species in southern (Samuelsen 1970) and northern Norway (Barnes et al. 2007). Our result is probably site-specific and was determined by the availability of suitable shells in the area or competition between sympatric hermit crab populations (Vance 1972; Bach et al. 1976; Abrams 1980). If P. bernhardus is indeed a better competitor for shells, it can rely mostly on the available shells of one, most preferred type, while the other crab species is forced to search across the remaining pool of shells of less favourable characteristics, so its shell selections are less focused. Indeed, the majority of individuals of P. bernhardus occupied the shells of Littorina spp. and both the experimental studies and in situ observations of P. bernhardus showed that Littorina spp. shells are strongly preferred over other types, such as Gibbula spp. shells (Elwood et al. 1979; Jackson & Elwood 1990).
The greatest diversity of shells (11 taxa) was used at the site located the furthest north (Smeerenburgfjorden). The greater richness in shells collected at Spitsbergen in the present study seems to be at odds with much more diverse gastropod species pools in northern Norway (133 species) compared to Spitsberen (93 species; Thorson 1944). Yet Barnes et al. (2007) demonstrated a lack of correlation between the latitude and number of shell types used despite the northward impoverishment of gastropods (Roy et al. 1996; Roy et al. 1998). Evidently, the richness of gastropod shells utilized by hermit crabs cannot be used as a surrogate for regional benthic diversity as was proposed, e.g., for the dead mollusc assemblages accumulated on sandy shores (Warwick & Light 2002).
Most of the gastropod species that were represented among the shells collected in this study occur in both Svalbard and continental Norwegian waters. All the species are included in a checklist of marine molluscs of the continental Norwegian waters (Høsæter 1986), except for Colus kroeyeri, Buccinum glaciale and B. polare, which are considered Arctic, circumpolar species that do not occur in Norway (Thorson 1944; Macpherson 1971; Bouchet & Waren 1985). A notable difference in the composition of the shells collected in the two regions was the absence of Littorina spp. shells at the sites in Svalbard. Litorina littorea, L. obtusata, Gibbula cineraria and Nucella lapillus are unlikely to occur in Svalbard waters though they are included in the list of marine invertebrate species recorded around Spitsbergen compiled by Palerud et al. (2004); their presence in Spitsbergen is not confirmed by other, more mollusc-oriented publications (Thorson 1944; Włodarska-Kowalczuk 2007) or by the IO PAN data archives based on 20 years investigating Svalbard benthic fauna (Włodarska-Kowalczuk et al., unpubl. data). In general, littorinids in the Arctic are markedly impoverished as compared to northern Norway. Studies of the Svalbard intertidal zone found only one periwinkle species: Littorina saxatilis (Węsławski et al. 1993; Węsławski et al. 1997). These studies also found that total faunal densities and biomass were much lower than in similar habitats (rocky shores) of northern Scandinavia.
Gastropod species richness at higher latitudes is much lower than in tropical or temperate regions, although 160 gastropod species occur in the Svalbard Archipelago (Palerud et al. 2004), and up to 38 species have been be found in Kongsfjorden, a single fjord in Spitsbergen (Włodarska-Kowalczuk 2007). Despite the variety of shells potentially available, most hermit crabs collected used only a few shell types. Typically large specimens of P. bernhardus inhabited shells of a large whelk B. undatum and the periwinkle L. littorea, while the smallest were found mostly in shells of G. cineraria. Although we did not perform any shell choice experiments, the fact that apart from the set of shells used by P. bernhardus, P. pubescens commonly inhabited Nucella lapillus might indicate that there is some interspecific competition in these hermit crabs and that by using an additional shell type some resource use overlap is minimized (Hazlett 1981). The lack of the largest specimens of P. pubescens in the collected samples further supports this (see discussion on crab size). Pagurus pubescens from the Svalbard Bank and Spitsbergen used mainly large Buccinidae shells and small shells of Cryptonatica affinis and Margarites groenlandicus. Two of these gastropod taxa (Buccinidae and M. groenlandicus) have been previously noted as the most frequently exploited by hermit crabs and highly abundant in the environment (Jensen & Bender 1973; Hazlett 1981; Hayward & Ryland 1999; Reiss et al. 2003), including the Arctic (Barnes et al. 2007; Kuklinski et al. 2008).
Undamaged gastropod shells of “optimal” weight, size and internal volume can offer good conditions for growth, reproduction (carrying eggs) and protection from desiccation, parasites, predators and other hermit crabs. It is generally considered that most hermit crab species prefer intact shells (Lancaster 1988), as a limited number of studies has demonstrated (Scully 1979; McClintock 1985; Wilber 1990; Pechenik & Lewis 2000). Such shells are a limiting resource (Vance 1972; Kellogg 1976; Hazlett 1981; Lancaster 1988; but see Barnes 1999) and some parts of hermit crab populations are forced to inhabit damaged shells. Large empty shells are often especially scarce in the environment (Lancaster 1988) and, in general, the larger the shell, the more likely it is to be broken (Barnes et al. 2007). Here, due to low sampling effort in some regions, the highest percentages of damaged shells (46–56%) were observed in the largest hermit crabs only at Spitsbergen. Predation is a major source of breakage (Zuschin et al. 2003). On the basis of species lists for northern Norway and Svalbard (Sandberg & McLaughlin 1998; Gulliksen et al. 1999; Gulliksen & Svensen 2004; Moen & Svensen 2004; Palerud et al. 2004), we can assume that the predation rates of shell-breaking and boring organisms (mammals, octopi, crabs, fish, gastropods, polychaetes) are similar in the studied regions. The proportion of damaged shells between northern Norway (31–34%), Spitsbergen (41%) and the Svalbard Bank (only 12%) is most likely related to the hydrodynamic regimes of those regions. High wave action in shallow waters and the rocky shores of Spitsbergen and northern Norway (PK, unpubl. data) results in higher abrasion and destruction of shells than in deeper offshore, relatively calmer Svalbard Bank.
Acknowledgements
The authors wish to thank Jakub Beszczynski for his underwater assistance, Joanna Pardus (IO PAN) for map preparation and two anonymous reviewers for their effort in improving the paper. The project was funded by the National Science Centre on the basis of decision DEC-2011/01/N/NZ8/04493 (PB). PK would like to acknowledge the funds (2022/LTSMP/2011/0) from the Polish Ministry of Science and Higher Education which enabled the completion of the study. We also acknowledge the Antoni Dębski Scholarship granted by the Polish Society of Hyperbaric Medicine and Technology (PTMiTH) to PB.
References
Abele
L.G. 1974.
Species diversity of decapod crustaceans in marine habitats. Ecology 55, 156–161.
Publisher Full Text
Abrams
P.A. 1980.
Resource partitioning and interspecific competition in a tropical hermit crab community. Ecology 46, 365–379.
Ayres-Peres
L.
&
Mantelatto
F.L. 2008.
Patterns of distribution of the hermit crab Loxopagurus loxochelis (Moreira, 1901) (Decapoda, Diogenidae) in two coastal areas of southern Brazil. Revista de Biología Marina y Oceanografía 43, 399–411.
Bach
C.,
Harlett
B.
&
Rittschof
D. 1976.
Effect of interspecific competition on fitness of the hermit crab Clibanarius tricolor. Ecology 57, 579–586.
Publisher Full Text
Bach
C.E.
&
Hazlett
B.A. 2009.
Shell shape affects movement patterns and microhabitat distribution in the hermit crabs Calcinus elegans, C. laevimanus and C. latens. Journal of Experimental Marine Biology and Ecology 382, 27–33.
Publisher Full Text
Balazy
P.
&
Kuklinski
P. 2013.
Mobile hard substrata—an additional biodiversity source in a high latitude shallow subtidal system. Estuarine, Coastal and Shelf Science 119, 153–161.
Publisher Full Text
Barnes
D.K.A. 1997.
Ecology of tropical hermit crabs at Quirimba Island, Mozambique: distribution, abundance and activity. Marine Ecology Progress Series 154, 133–142.
Publisher Full Text
Barnes
D.K.A. 1999.
Ecology of tropical hermit crabs at Quirimba Island, Mozambique: shell characteristics and utilization. Marine Ecology Progress Series 183, 241–251.
Publisher Full Text
Barnes
D.K.A.
&
De Grave
S. 2000.
Ecology of tropical hermit crabs at Quirimba Island, Mozambique: niche width and resource allocation. Marine Ecology Progress Series 206, 171–179.
Publisher Full Text
Barnes
D.K.A.
&
Kukliński
P. 2005.
Low colonisation on artificial substrata in Arctic Spitsbergen. Polar Biology 29, 65–69.
Publisher Full Text
Barnes
D.K.A.,
Kuklinski
P.
&
Włodarska-Kowalczuk
M. 2007.
Richness, abundance and shell use of Subarctic and Arctic hermit crabs. Marine Biology 152, 1133–1142.
Publisher Full Text
Bell
J.J. 2005.
Influence of occupant microhabitat on the composition of encrusting communities on gastropod shells. Marine Biology 147, 653–661.
Publisher Full Text
Berge
J.,
Johnsen
G.,
Nilsen
F.,
Gulliksen
B.
&
Slagstad
D. 2005.
Ocean temperature oscillations enable reappearance of blue mussels Mytilus edulis in Svalbard after a 1000 year absence. Marine Ecology Progress Series 303, 167–175.
Publisher Full Text
Berge
J.,
Renaud
P.,
Eiane
K.,
Gulliksen
B.,
Cottier
F.,
Varpe
O.
&
Brattegard
T. 2009.
Changes in the decapod fauna of an Arctic fjord during the last 100 years (1908–2007). Polar Biology 32, 953–961.
Publisher Full Text
Bertness
M.D. 1981a.
The influence of shell-type on hermit crab growth rate and clutch size (Decapoda, Anomura). Crustaceana 40, 197–205.
Publisher Full Text
Birula
A. 1907.
Zoologische Ergebnisse der Russischen Expedition nach Spitzbergen. Crustacea–Decapoda. (Zoological results of the Russian expedition to Spitsbergen. Crustacea–Decapoda.) Annuairedu Musée Zoologique de l'Académie Impériale des Sciences de St. Pétersbourg 11, 1–68.
Bookhout
C.G. 1964.
Salinity effects on larval development of Pagurus bernhardus (L.) reared in the laboratory. Ophelia 1, 275–294.
Publisher Full Text
Bouchet
P.
&
Waren
A. 1985.
Revision of the northeast Atlantic bathyal and abyssal Neogastropoda excluding Turridae (Mollusca, Gastropoda). Bolletino Malacologico Suppl. 1, 123–296.
Brattegard
T.
&
Holthe
T. (eds.) 2001. Distribution of marine, benthic macroorganisms in Norway. A tabulated catalogue. Research report 2001–3.
Trondheim: Directorate for Nature Management.
Chapelle
G.
&
Peck
L.S. 1999.
Polar gigantism dictated by oxygen availability. Nature 399, 114–115.
Publisher Full Text
Chapelle
G.
&
Peck
L.S. 2004.
Amphipod crustacean size spectra: new insights in the relationship between size and oxygen. Oikos 106, 167–175.
Publisher Full Text
Christiansen
M.E.
&
Christiansen
B.O. 1962. The Crustacea Decapoda of Isfjorden: a comparison with the Swedish Spitsbergen Expedition in 1908. Acta Scientia 19. Tromsø, Norway: Tromsø, Museum.
Clarke
K.R.
&
Gorley
R.N. 2001. PRIMER v6: user manual/tutorial. Plymouth: PRIMER-E.
Core Writing Team, Pachauri
R.K.
&
Reisinger
A. (eds.) 2007. Climate change 2007: synthesis report. A report of the Intergovernmental Panel on Climate Change.
Geneva: Intergovernmental Panel on Climate Change.
Davenport
J. 1972.
Volume changes shown by some littoral Anomuran Crustacea. Journal of the Marine Biological Association of the United Kingdom 52, 863.
Publisher Full Text
Dawirs
R.R. 1979.
Effects of temperature and salinity on larval development of Pagurus bernhardus (Decapoda, Paguridae). Marine Ecology Progress Series 1, 323–329.
Publisher Full Text
Dawirs
R.R. 1981.
Elemental composition (C, N, H) and energy in the development of Pagurus bernhardus (Decapoda: Paguridae) megalopa. Marne Biology 64, 117–123.
Publisher Full Text
Dawson
T.P.,
Jackson
S.T.,
House
J.I.,
Prentice
I.C.
&
Mace
G.M. 2011.
Beyond predictions: biodiversity conservation in a changing climate. Science 332, 53–58.
PubMed Abstract | Publisher Full Text
Duarte
C.M.,
Agusti
S.,
Wassmann
P.,
Arrieta
J.M.,
Alcaraz
M.,
Coello
A.,
Marbà
N.,
Hendriks
I.E.,
Holding
J.,
García-Zarandona
I.,
Kritzberg
E.
&
Vaqué
D. 2012.
Tipping elements in the Arctic marine ecosystem. Ambio 41, 44–55.
PubMed Abstract | PubMed Central Full Text | Publisher Full Text
Duris
Z. 1995. Decapod crustaceans collected in Norwegian and Spitsbergen waters during the summer cruise of the R/V Oceania, 1995. In
J.
Repelewska-Pękalowa
&
K.
Pękala (eds.): Spitsbergen geographical expeditions. Polar session. Problems of the contemporaneous and Pleistocene periglacial zone. Pp. 128–132.
Lublin,
Poland: Institute of Earth Sciences, Maria Curie-Skłodoska University.
Elverhøi
A.,
Lonne
O.
&
Seland
R. 1983.
Glaciomarine sedimentation in a modern fjord environment, Spitsbergen. Polar Research 1, 127–150.
Publisher Full Text
Elverhøi
A.
&
Solheim
A. 1983. The physical environment—western Barents Sea. 1:1500000, sheet A: surface sediment distribution. Norsk Polarinstitutt Skrifter 179A. Tromsø: Norwegian Polar Institute.
Elwood
R.W.,
Mark
N.
&
Dick
J.T.A. 1995.
Consequences of shell-species preferences for female reproduction success in the hermit crab Pagurus bernhardus. Marine Biology 123, 431–434.
Publisher Full Text
Elwood
R.W.,
McClean
A.
&
Webb
L. 1979.
The development of shell preferences by the hermit crab Pagurus bernhardus. Animal Behaviour 27, 940–946.
Publisher Full Text
Eriksson
S.,
Evans
S.
&
Tallmark
B. 1975.
On the co-existence of scavengers on shallow, sandy bottoms of Gullmar Fjord (Sweden): adaptations to substratum, temperature and salinity. Zoon 3, 65–70.
Falk-Petersen
I.B.,
Kanapathippilai
P.,
Primicerio
R.
&
Hansen
T.K. 2010.
Size, locality and seasonally related feeding preferences of common wolffish (Anarhichas lupus L.) from north-Norwegian waters. Marine Biology Research 6, 201–212.
Publisher Full Text
Fay
F.H.
&
Burns
J.J. 1988.
Maximal feeding depth of walruses. Arctic 41, 239–240.
Publisher Full Text
Fransozo
A.,
Bertini
G.,
Braga
A.A.
&
Negreiros-Fransozo
M.L. 2007.
Ecological aspects of hermit crabs (Crustacea, Anomura, Paguroidea) off the northern coast of São Paulo State, Brazil. Aquatic Ecology 42, 437–448.
Publisher Full Text
Fransozo
A.,
Mantelatto
F.L.M.,
Bertini
G.,
Fernandes-Goes
L.C.
&
Martinelli
J.M. 1998.
Distribution and assemblages of anomuran crustaceans in Ubatuba Bay, north coast of Sao Paulo State, Brazil. Acta Biologica Venezuelica 18, 17–25.
Gerlach
S.A.,
Ekstrøm
D.K.
&
Eckardt
P.B. 1976.
Filter feeding in the hermit crab. Oecologia 24, 257–264.
Publisher Full Text
Greenwood
J.G. 1972.
The mouthparts and feeding behaviour of two species of hermit crabs. Journal of Natural History 6, 325–337.
Publisher Full Text
Gulliksen
B.,
Palerud
R.,
Brattegard
T.
&
Sneli
J. (eds.) 1999. Distribution of marine benthic macro-organisms at Svalbard (including Bear Island) and Jan Mayen. Research Report for DN 1999–4.
Trondheim: Directorate for Nature Management.
Gulliksen
B.
&
Svensen
E. 2004. Svalbard and life in polar oceans. Kristiansund, Norway: Kom Publishing.
Harms
J. 1992.
Larval development and delayed metamorphosis in the hermit crab Cllbanarius erythropus (Latrielle) (Crustacea, Diogenidae). Journal of Experimental Marine Biology and Ecology 156, 151–160.
Publisher Full Text
Harvey
A. 1996.
Delayed metamorphosis in Florida hermit crabs: multiple cues and constraints (Crustacea: Decapoda: Paguridae and Diogenidae). Marine Ecology Progress Series 141, 27–36.
Publisher Full Text
Hayward
P.J.
&
Ryland
J.S. (eds.) 1999. The marine fauna of the British Isles and north-west Europe. Vol. 1: introduction and protozoans to arthropods.
Oxford: Oxford University Press.
Hazlett
B.A. 1981.
The behavioural ecology of hermit crabs. Annual Review of Ecology, Evolution, and Systematics 12, 1–22.
Publisher Full Text
Heegaard
P.E. 1941. Decapod crustaceans. In
M.
Degerbel (ed.): The zoology of east Greenland. Meddelelser om Grønland 121. Pp. 1–72.
Copenhagen: Commission for Scientific Investigations in Greenland.
Henrich
R.,
Freiwald
A.,
Bickert
T.
&
Schafer
P. 1997. Evolution of an Arctic open-shelf carbonate platform, Spitsbergen Bank (Barents Sea). In
N.P.
James
&
J.A.D.
Clarke (eds.): Cool-water carbonates. Pp. 163–181.
Tulsa: Society for Sedimentary Geology.
Hop
H.,
Pearson
T.,
Hegseth
E.N.,
Kovacs
K.M.,
Wiencke
C.,
Kwasniewski
S.,
Eiane
K.,
Mehlum
F.,
Gulliksen
B.,
Włodarska-Kowalczuk
M.,
Lydersen
C.,
Węsławski
J.M.,
Cochrane
S.,
Gabrielsen
G.W.,
Leakey
R.J.G.,
Lønne
O.J.,
Zajaczkowski
M.,
Falk-Petersen
S.,
Kendall
M.,
Wängberg
S.A.,
Bischof
K.,
Voronkov
A.Y.,
Kovaltchouk
N.A.,
Wiktor
J.,
Poltermann
M.,
di Prisco
G.,
Papucci
C.
&
Gerland
S. 2002.
The marine ecosystem of Kongsfjorden, Svalbard. Polar Research 21, 167–208.
Publisher Full Text
Høsæter
T. 1986.
An annotated check-list of marine molluscs of the Norwegian coast and adjacent waters. Sarsia 71, 73–145.
Hurlbert
S.H. 1971.
The non-concept of species diversity; a critique and alternative parameters. Ecology 52, 577–586.
Publisher Full Text
Ingle
R.W. 1993. Hermit crabs of the northeastern Atlantic Ocean and the Mediterranean Sea: an illustrated key. London: Chapman & Hall.
Jackson
H.G. 1913.
Eupagurus. Liverpool Marine Biology Committee Memoirs 21, 1–79.
Jackson
N.W.
&
Elwood
R.W. 1990.
Interrupting an assessment process to probe changes in the motivational state. Animal Behaviour 39, 1068–1077.
Publisher Full Text
Jakubas
D.,
Głuchowska
M.,
Wojczulanis-Jakubas
K.,
Karnovsky
N.,
Keslinka
L.,
Kidawa
D.,
Walkusz
W.,
Boehnke
R.,
Cisek
M.,
Kwaśniewski
S.
&
Lech Stempniewicz
L. 2011.
Foraging effort does not influence body condition and stress level in little auks. Marine Ecology Progress Series 432, 277–290.
Publisher Full Text
Jensen
K.
&
Bender
K. 1973.
Invertebrates associated with snail shells inhabited by Pagurus bernhardus (L.) (Decapoda). Ophelia 10, 185–192.
Publisher Full Text
Jones
C.G.,
Lawton
J.H.
&
Shachak
M. 1994.
Organisms as ecosystem engineers. Oikos 69, 373–386.
Publisher Full Text
Jones
C.G.,
Lawton
J.H.
&
Shachak
M. 1997.
Positive and negative effects of organisms as physical ecosystem engineers. Ecology 78, 1946–1957.
Kaczmarek
H.,
Włodarska-Kowalczuk
M.,
Legeżyńska
J.
&
Zajaczkowski
M. 2005.
Shallow sublittoral macrozoobenthos in Kongsfjord, west Spitsbergen, Svalbard. Polish Polar Research 26, 137–155.
Kędra
M.,
Renaud
P.E.,
Andrade
H.,
Goszczko
I.
&
Ambrose
W.G. 2013.
Benthic community structure, diversity, and productivity in the shallow Barents Sea bank (Svalbard Bank). Marine Biology 160, 805–819.
Publisher Full Text
Kędra
M.,
Włodarska-Kowalczuk
M.
&
Węsławski
J.M. 2010.
Decadal change in soft-bottom community structure in High Arctic fjord (Kongsfjord, Svalbard). Polar Biology 33, 1–11.
Kellogg
C.W. 1976.
Gastropod shells: a potentially limiting resource for hermit crabs. Journal of Experimental Marine Biology and Ecology 22, 101–111.
Publisher Full Text
Kuklinski
P.,
Barnes
D.K.A.
&
Włodarska-Kowalczuk
M. 2008. Gastropods shells, hermit crabs and Arctic bryozoan richness. In
S.J.
Hageman
et al. (eds.): Bryozoan Research 2007: Proceedings of the 14th International Bryozoology Association Conference, Boone, North Carolina, July 1–8, 2007. Virginia Museum of Natural History Special Publication 15. Pp. 93–100.
Martinsville,
VA: Virginia Museum of Natural History.
Kwasniewski
S.,
Gluchowska
M.,
Jakubas
D.,
Wojczulanis-Jakubas
K.,
Walkusz
W.,
Karnovsky
N.,
Blachowiak-Samolyk
K.,
Cisek
M.
&
Stempniewicz
L. 2010.
The impact of different hydrographic conditions and zooplankton communities on provisioning little auks along the west coast of Spitsbergen. Progress in Oceanography 87, 72–82.
Publisher Full Text
Kwasniewski
S.,
Gluchowska
M.,
Walkusz
W.,
Karnovsky
N.J.,
Jakubas
D.,
Wojczulanis-Jakubas
K.,
Harding
A.M.A.,
Goszczko
I.,
Cisek
M.,
Beszczynska-Möller
A.,
Walczowski
W.,
Węsławski
J.M.
&
Stempniewicz
L. 2012.
Interannual changes in zooplankton on the west Spitsbergen Shelf in relation to hydrography and their consequences for the diet of planktivorous seabirds. ICES Journal of Marine Science 69, 890–901.
Publisher Full Text
Lancaster
I. 1988. Pagurus bernhardus (L.)—an introduction to the natural history of hermit crabs. Field Studies 7, 189–238.
Lefauconnier
B.,
Hagen
J.O.
&
Rudant
J.P. 1994.
Flow speed and calving rate of Kongsbreen glacier, 70°N Spitsbergen, Svalbard, using SPOT images. Polar Research 13, 59–66.
Publisher Full Text
Lindley
J.A. 1998.
Diversity, biomass and production of decapod crustacean larvae in a changing environment. Invertebrate Reproduction & Development 33, 209–219.
PubMed Abstract | PubMed Central Full Text | Publisher Full Text
Linnane
A.,
Ball
B.,
Munday
B.,
Browne
R.
&
Mercer
J.P. 2002.
Faunal description of an Irish cobble site using airlift suction sampling. Proceedings of the Royal Irish Academy Section B 103, 41–48.
Publisher Full Text
Loeng
H. 1991.
Features of the physical oceanographic conditions of the Barents Sea. Polar Research 10, 5–18.
Publisher Full Text
Lydersen
C.,
Gjertz
I.
&
Węsławski
J.M. 1989.
Stomach contents of autumn-feeding marine vertebrates from Hornsund, Svalbard. Polar Record 25, 107–114.
Publisher Full Text
Macpherson
E. 1971.
The marine Mollusca of Arctic Canada. Publications in Biological Oceanography 3, 149.
Magdziarz
K. 1984. Rozmieszczenie Decapoda w fjordzie Hornsund, Spitsbergen. (Distribution of Decapoda in Hornsund fjord, Spitsbergen.) MSc thesis, University of Gdansk.
McClintock
T.S. 1985.
Effects of shell condition and size upon the shell choice behavior of a hermit crab. Journal of Experimental Marine Biology and Ecology 88, 271–285.
Publisher Full Text
McLean
R. 1983.
Gastropod shells: a dynamic resource that helps shape benthic community structure. Journal of Experimental Marine Biology and Ecology 69, 151–174.
Publisher Full Text
Moen
F.E.
&
Svensen
E. 2004. Marine fish & invertebrates of northern Europe. Kristiansund, Norway: Kom Publishing.
Moksnes
P.O. 2002.
The relative importance of habitat-specific settlement, predation and juvenile dispersal for distribution and abundance of young juvenile shore crabs Carcinus maenas L. Journal of Experimental Marine Biology and Ecology 271, 41–73.
Publisher Full Text
NAMMCO (North Atlantic Marine Mammal Commission). 1997. Report of the Scientific Committee. NAMMCO annual report 1996. P. 166.
Tromsø,
Norway: NAMMCO.
Palerud
R.,
Gulliksen
B.,
Brattegard
T.,
Sneli
J.A.
&
Vader
W. 2004. The marine macro-organisms in Svalbard waters. In
P.
Prestrud
et al. (eds.): Norsk Polarinstitutt Skrifter 201. A catalogue of the terrestrial and marine animals of Svalbard. Pp. 5–57.
Tromsø: Norwegian Polar Institute.
Pallas
A.,
Garcia-Calvo
B.,
Corgos
A.,
Bernardez
C.
&
Freire
J. 2006.
Distribution and habitat use patterns of benthic decapod crustaceans in shallow waters: a comparative approach. Marine Ecology Progress Series 324, 173–184.
Publisher Full Text
Pechenik
J.A.
&
Lewis
S. 2000.
Avoidance of drilled gastropod shells by the hermit crab Pagurus longicarpus at Nahant, Massachusetts. Journal of Experimental Marine Biology and Ecology 253, 17–32.
PubMed Abstract | Publisher Full Text
Peura
J.F.,
Lovvorn
J.R.,
North
C.A.
&
Kolts
J.M. 2013.
Hermit crab population structure and association with gastropod shells in the northern Bering Sea. Journal of Experimental Marine Biology and Ecology 449, 10–16.
Publisher Full Text
Reiss
H.,
Knauper
S.
&
Kroncke
I. 2003.
Invertebrate associations with gastropod shells inhabited by Pagurus bernhardus (Paguridae) secondary hard substrate increasing biodiversity in North Sea soft-bottom communities. Sarsia 88, 404–415.
Publisher Full Text
Renaud
P.E.,
Carroll
M.L.
&
Ambrose
W.G.J. 2008. Effects of global warming on Arctic sea-floor communities and its consequences for higher trophic levels. In
C.M.
Duarte (ed.): Impacts of global warming on polar ecosystems. Pp. 141–177.
Bilbao: Fundación BBVA.
Renaud
P.E.,
Włodarska-Kowalczuk
M.,
Trannum
H.,
Holte
B.,
Węsławski
J.M.,
Cochrane
S.,
Dahle
S.
&
Gulliksen
B. 2007.
Multidecadal stability of benthic community structure in a High-Arctic glacial fjord (Van Mijenfjord, Spitsbergen). Polar Biology 30, 295–305.
Publisher Full Text
Roberts
M.H. 1971.
Larval development of Pagurus longicarpus Say reared in the laboratory. II. Effects of reduced salinity on larval development. The Biological Bulletin 140, 104–116.
PubMed Abstract | Publisher Full Text
Robinson
M.
&
Tully
O. 2000.
Spatial variability in decapod community structure and recruitment in sub-tidal habitats. Marine Ecology Progress Series 191, 133–141.
Publisher Full Text
Roy
K.,
Jablonski
D.
&
Valentine
J.W. 1996.
Higher taxa in biodiversity studies: patterns from eastern Pacific marine molluscs. Philosophical Transactions of the Royal Society B 351, 1605–1613.
Publisher Full Text
Roy
K.,
Jablonski
D.,
Valentine
J.W.
&
Rosenberg
G. 1998.
Marine latitudinal diversity gradients: tests of causal hypotheses. Proceedings of the National Academy of Sciences of the United States of America 95, 3699–3702.
PubMed Abstract | PubMed Central Full Text | Publisher Full Text
Sakshaug
E.,
Johnsen
G.,
Kristiansen
S.,
von Quillfeldt
C.,
Rey
F.,
Slagstad
D.
&
Thingstad
F. 2009. Phytoplankton and primary production. In
E.
Sakshaug
et al. (eds.): Ecosystem Barents Sea.
Trondheim: Tapir Academic Press.
Samuelsen
T.J. 1970.
The biology of six species of Anomura (Crustacea, Decapoda) from Raunefjorden, western Norway. Sarsia 45, 25–52.
Sandberg
L.
&
McLaughlin
P.A. 1998. Crustacea, Decapoda, Paguridea. Marine invertebrates of Scandinavia 10.
Oslo: Scandinavian University Press.
Sandford
F. 2003.
Population dynamics and epibiont associations of hermit crabs (Crustacea: Decapoda: Paguroidea) on Dog Island, Florida. Memoirs of Museum Victoria 60, 45–52.
Scully
E.P. 1979.
The effects of gastropod shell availability and habitat characteristics on shell utilization the intertidal hermit crab Pagurus longicarpus Say. Journal of Experimental Marine Biology and Ecology 37, 139–152.
Publisher Full Text
Selbie
C.M. 1921.
The Decapoda Reptantia of the coasts of Ireland. Part II: Paguridea. Ireland Fisheries Branch, Scientific Investigations 1, 1–68.
Sokolov
V.
2006. Zoogeography of decapod crustaceans in the Euro-Asiatic seas of Arctic region.
Paper presented at the International Council for the Exploration of the Sea annual science conference, theme session D. 19–23 September, Maastricht, Netherlands.
Squires
H.J.,
Ennis
G.
&
Dawe
G. 1993.
On biology of two sympatric species of hermit crab at St. Chads, Newfoundland. NAFO Scientific Council Studies 34, 7–17.
Stachowitsch
M. 1977. The hermit crab micro biocenosis—the role of mobile secondary hard bottom elements in a north Adriatic benthic community. In
B.F.
Keegan (ed.): Biology of benthic organisms. Proceedings of the European Symposium in Marine Biology. Pp. 549–558.
Oxford: Pergamon Press.
Stelle
M.A. 1999.
Effects of shelter and predators on reef fishes. Journal of Experimental Marine Biology and Ecology 233, 65–79.
Publisher Full Text
Svendsen
H.,
Beszczynska-Møller
A.,
Hagen
J.O.,
Lefauconnier
B.,
Tverberg
V.,
Gerland
S.,
Ørbæk
J.B.,
Bischof
K.,
Papucci
C.,
Zajaczkowski
M.,
Azzolini
R.,
Bruland
O.,
Wiencke
C.,
Winther
J.-G.
&
Dallmann
W. 2002.
The physical environment of Kongsfjorden–Krossfjorden, and Arctic fjord system in Svalbard. Polar Research 21, 133–166.
Publisher Full Text
Swerpel
S. 1985.
The Hornsund fiord: water masses. Polish Polar Research 6, 475–496.
Thorson
G. 1944. The zoology of East Greenland. Marine Gastropoda Prosobranchia. Meddelelser om Grønland 121.
Copenhagen: Commission for Scientific Investigations in Greenland.
Thorson
G. 1946. Reproduction and larval development of Danish marine bottom invertebrates. Meddelelser fra Kommissionen for Danmarks Fiskeri- og Havundersøgelser Serie Plankton 4.
Copenhagen: Commission for Danish Fishery and Marine Research.
Tricarico
E.,
Bertocchi
S.,
Brusconi
S.,
Antonio
L.
&
Gherardi
F. 2009.
Shell recruitment in the Mediterranean hermit crab Clibanarius erythropus. Journal of Experimental Marine Biology and Ecology 381, 42–46.
Publisher Full Text
Vance
R.R. 1972.
Competition and mechanism of coexistence in three sympatric species of intertidal hermit crabs. Ecology 53, 1062–1074.
Publisher Full Text
Wahle
R.A.
&
Steneck
R.S. 1991.
Recruitment habitats and nursery grounds of the American lobster Homarus americanus: a demographic bottleneck? Marine Ecology Progress Series 69, 231–243.
Publisher Full Text
Walczowski
W.
&
Piechura
J.
2006. New evidence of warming propagating toward the Arctic Ocean. Geophysical Research Letters
33, L12601, doi: 10.1029/2006GL025872.
Publisher Full Text
Walczowski
W.,
Piechura
J.,
Goszczko
I.
&
Wieczorek
P. 2012.
Changes in Atlantic water properties: an important factor in the European Arctic marine climate. ICES Journal of Marine Science 69, 864–869.
Publisher Full Text
Wang
M.
&
Overland
J.E.
2012. A sea ice free summer Arctic within 30 years: an update from CMIP5 models. Geophysical Research Letters
39, L18501, doi: 10.1029/2012GL052868.
Publisher Full Text
Warner
G.F. 1977. The biology of crabs. London: Elek.
Warwick
R.M.,
Emblow
C.,
Feral
J.P.,
Hummel
H.,
van Avesaath
P.
&
Heip
C. 2003. European marine biodiversity research sites: report of the European Concerted Action: BIOMARE implementation and networking of large scale, long term marine biodiversity research in Europe.
Yerseke,
Netherlands: Centre for Estuarine and Marine Research, Ecology Netherlands Institute for Ecological Research.
Warwick
R.M.
&
Light
J. 2002.
Death assemblages of Molluscs on St Martin's Flats, Isles of Scilly: a surrogate for regional biodiversity? Biodiversity & Conservation 11, 99–112.
PubMed Abstract | PubMed Central Full Text | Publisher Full Text
Wassmann
P.,
Duarte
C.M.,
Agusti
S.
&
Sejr
M.K. 2011.
Footprints of climate change in the Arctic marine ecosystem. Global Change Biology 17, 1235–1249.
Publisher Full Text
Węsławski
J.M. 1987.
Distribution of Decapoda (Crustacea) in south Spitsbergen coastal waters with remarks on their ecology and breeding biology. Polish Polar Research 8, 121–134.
Węsławski
J.M.,
Kendall
M.,
Włodarska-Kowalczuk
M.,
Iken
K.,
Kędra
M.,
Legeżyńska
J.
&
Sejr
M.K. 2011.
Climate change effects on Arctic fjord and coastal macrobenthic diversity—observations and predictions. Marine Biodiversity 41, 71–85.
Publisher Full Text
Węsławski
J.M.,
Koszteyn
J.,
Kwaśniewski
S.,
Stempniewicz
L.
&
Malinga
M. 1999.
Summer food resources of the little auk, Alle alle (L.) in the European Arctic seas. Polish Polar Research 20, 387–403.
Węsławski
J.M.,
Kwasniewski
S.
&
Wiktor
J. 1991.
Winter in a Svalbard fjord ecosystem. Arctic 44, 115–123.
Węsławski
J.M.,
Stempniewicz
L.,
Mehlum
F.
&
Kwasniewski
S. 1999.
Summer feeding strategy of the little auk (Alle alle) from Bjornoya, Barents Sea. Polar Biology 21, 129–134.
Publisher Full Text
Węsławski
J.M.,
Wiktor
J.,
Jr.
&
Kotwicki
L. 2010.
Increase in biodiversity in the Arctic rocky littoral, Sorkappland, Svalbard, after 20 years of climate warming. Marine Biodiversity 40, 123–130.
Publisher Full Text
Węsławski
J.M.,
Wiktor
J.,
Zajaczkowski
M.
&
Swerpel
S. 1993.
Intertidal zone of Svalbard 1. Macroorganisms distribution and biomass. Polar Biology 13, 73–108.
Węsławski
J.M.,
Zajączkowski
M.,
Kwaśniewski
S.,
Jezierski
J.
&
Moskal
W. 1988.
Seasonality in an Arctic fjord ecosystem: Hornsund, Spitsbergen. Polar Research 6, 185–189.
Publisher Full Text
Węsławski
J.M.,
Zajączkowski
M.,
Wiktor
J.
&
Szymelfenig
M. 1997.
Intertidal zone of Svalbard 3. Littoral of a Subarctic, oceanic island: Bjornoya. Polar Biology 18, 45–52.
Publisher Full Text
Weydmann
A.,
Søreide
J.E.,
Kwasniewski
S.,
Leu
E.,
Falk-Petersen
S.
&
Berge
J. 2013.
Ice-related seasonality in zooplankton community composition in a High Arctic fjord. Journal of Plankton Research 35, 831–842.
Publisher Full Text
Wilber
T.P. 1990.
Influence of size, species and damage on shell selection by the hermit crab Pagurus longicarpus. Marine Biology 104, 31–39.
Publisher Full Text
Williams
J.D.
&
McDermot
J.J. 2004.
Hermit crab biocoenoses: a worldwide review of the diversity and natural history of hermit crab associates. Journal of Experimental Marine Biology and Ecology 305, 1–128.
Publisher Full Text
Winkelmann
D.
&
Knies
J.
2005. Recent distribution and accumulation of organic carbon on the continental margin west off Spitsbergen. Geochemistry, Geophysics, Geosystems
6, Q09012, doi: 10.1029/2005GC000916.
Publisher Full Text
Włodarska-Kowalczuk
M. 2007.
Molluscs in Kongsfjorden (Spitsbergen, Svalbard): a species list and patterns of distribution and diversity. Polar Research 26, 48–63.
Publisher Full Text
Włodarska-Kowalczuk
M.,
Kendall
M.,
Węsławski
J.M.,
Klages
M.
&
Soltwedel
T. 2004.
Depth gradients of benthic standing stock and diversity on the continental margin at a high-latitude ice-free site (off Spitsbergen, 79°N). Deep-Sea Research Part I 51, 1903–1914.
Publisher Full Text
Włodarska-Kowalczuk
M.,
Pearson
T.H.
&
Kendall
M.A. 2005.
Benthic response to chronic natural physical disturbance by glacial sedimentation in an Arctic fiord. Marine Ecology Progress Series 303, 31–34.
Publisher Full Text
Włodarska-Kowalczuk
M.,
Szymelfenig
M.
&
Zajaczkowski
M. 2007.
Dynamic sedimentary environments of an Arctic glacier-fed river estuary (Adventfjorden, Svalbard). II. Meio- and macrobenthic fauna. Estuarine, Coastal and Shelf Science 74, 274–284.
Publisher Full Text
Włodarska-Kowalczuk
M.,
Węslawski
J.M.
&
Kotwicki
L. 1998.
Spitsbergen glacial bays macrobenthos—a comparative study. Polar Biology 20, 66–73.
Publisher Full Text
Yoshino
K.,
Goshima
S.
&
Nakao
S. 2002.
Temporal reproductive patterns within a breeding season of the hermit crab Pagurus filholi: effects of crab size and shell species. Marine Biology 141, 1069–1075.
Publisher Full Text
Zajączkowski
M.
&
Włodarska-Kowalczuk
M. 2007.
Dynamic sedimentary environments of an Arctic glacier-fed river estuary (Adventfjorden, Svalbard). I. Flux, deposition and dynamics of the sediment. Estuarine, Coastal and Shelf Science 74, 285–296.
Publisher Full Text
Zuschin
M.
&
Pille
W.E. 2007.
Gastropod shells recycled—an example from a rocky tidal flat in the northern Red Sea. Lethaia 30, 127–134.
Publisher Full Text
Zuschin
M.,
Stachowitsch
M.
&
Stanton
R.J. 2003.
Patterns and processes of shell fragmentation in modern and ancient marine environments. Earth-Science Reviews 63, 33–82.
Publisher Full Text