RESEARCH/REVIEW ARTICLE
Distribution and diversity of Tardigrada along altitudinal gradients in the Hornsund, Spitsbergen (Arctic)
Krzysztof Zawierucha,1 Jerzy Smykla,2,3 Łukasz Michalczyk,4 Bartłomiej Gołdyn5,6 & Łukasz Kaczmarek1,6
1 Department of Animal Taxonomy and Ecology, Faculty of Biology, Adam Mickiewicz University in Poznań, Umultowska 89, PL-61-614 Poznań, Poland
2 Department of Biodiversity, Institute of Nature Conservation, Polish Academy of Sciences, Mickiewicza 33, PL-31-120 Kraków, Poland
3 Present address: Department of Biology and Marine Biology, University of North Carolina, Wilmington, 601 S. College Rd., Wilmington, NC 28403, USA
4 Department of Entomology, Institute of Zoology, Jagiellonian University, Gronostajowa 9, PL-30-387 Kraków, Poland
5 Department of General Zoology, Faculty of Biology, A. Mickiewicz University in Poznań, Umultowska 89, PL-61-614 Poznań, Poland
6 Laboratorio de Ecología Natural y Aplicada de Invertebrados, Universidad Estatal Amazónica, Puyo, Ecuador
Abstract
Two transects were established and sampled along altitudinal gradients on the slopes of Ariekammen (77°01′N; 15°31′E) and Rotjesfjellet (77°00′N; 15°22′E) in Hornsund, Spitsbergen. In total 59 moss, lichen, liverwort and mixed moss–lichen samples were collected and 33 tardigrade species of Hetero- and Eutardigrada were found. The α diversity ranged from 1 to 8 per sample; the estimated number of species based on all analysed samples was 52±17 for the Chao 2 estimator and 41 for the incidence-based coverage estimator. According to the results of detrended canonical correspondence analysis, altitude and type of substratum were the most important factors influencing tardigrade communities in the investigated area. Macrobiotus crenulatus, M. hufelandi hufelandi and Hypsibius pallidus dominated in the lower elevations, whereas Echiniscus wendti and E. merokensis merokensis prevailed in samples from higher plots. Macrobiotus islandicus islandicus was collected most often from mosses collected from rock whereas Isohypsibius coulsoni from mosses collected from soil. Analyses of covariance were employed to test for differences in species richness between the transects in relation to altitude. Contrary to expectations, there were significant differences in species richness between the transects, but richness was not significantly related to altitude. Interestingly, significant effects of colonies of seabirds, little auk (Alle alle), on the tardigrades communities were detected. Additionally, in one of the samples first ever males of Milnesium asiaticum were found. Their measurements and microphotographs are provided herein.
Keywords
Arctic; biodiversity; climate change; invertebrate ecology; Milnesium; Tardigrada.
Correspondence
Krzysztof Zawierucha, Department of Animal Taxonomy and Ecology, Faculty of Biology, Adam Mickiewicz University in Poznań, Umultowska 89, PL-61-614 Poznań, Poland.
E-mail: k.p.zawierucha@gmail.com
To access the supplementary material for this article, please see
supplementary files under Article Tools Online.
(Published: 2 February 2015)
Polar Research 2015. © 2015 K. Zawierucha et al. This is an Open Access article distributed under the terms of the Creative Commons Attribution-Noncommercial 3.0 Unported License (http://creativecommons.org/licenses/by-nc/3.0/), permitting all non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Citation: Polar Research 2015, 34, 24168, http://dx.doi.org/10.3402/polar.v34.24168
Tardigrades, commonly known as water bears, are micrometazoans typically less than 1 mm in length. They are distributed across all continents, inhabiting a great majority of terrestrial, freshwater and marine ecosystems, from the highest mountain peaks and polar deserts to the deep ocean floors. They are famous for their impressive cryptobiotic properties that allow them to withstand extreme conditions, including these of the outer space (Ramazzotti & Maucci 1983; Bertolani et al. 2004; Guil 2008; Guidetti et al. 2011; Wełnicz et al. 2011). In extreme environments on Earth, such as those of the polar regions, tardigrades usually live alongside bdelloid rotifers and nematodes (e.g., Sohlenius & Boström 2008; Smykla et al. 2010), but in some cases they may constitute the only metazoan element present in an ecosystem (e.g., Convey & McInnes 2005).
Although widely distributed and an important element of extreme ecosystems, tardigrades for decades have been largely neglected and are therefore poorly known. Only in recent years, there has been an increasing interest in assessing their diversity and identifying factors determining their distribution. As a consequence, within the last two decades the number of described tardigrade species has doubled; at present, ca. 1200 species are known globally (Degma et al. 2011; Vicente & Bertolani 2013). However, this number is probably still severely underestimated (Guil & Cabrero-Sanudo 2007). In spite of the increased interest in tardigrades and the growing literature on them, knowledge of their distribution, abundance and diversity along environmental and geographical gradients is still very fragmentary (e.g., McInnes & Pugh 2007; Garey et al. 2008).
The tardigrade fauna of Spitsbergen has a long history of investigations, and compared to the state of knowledge on global water bear diversity, it seems to be relatively well investigated. The first studies of Svalbard’s tardigrades took in the late 19th century (Scourfield 1897) and were continued by a number of researchers throughout the following century (for a summary see Zawierucha et al. 2013 and literature cited therein). However, despite numerous investigations of the tardigrades of Svalbard, the majority of publications were limited to faunistic reports and descriptions of new taxa (e.g., Zawierucha et al. 2013 and literature cited therein). Until now, only Dastych (1985), apart from providing a faunistic report, also analysed ecological factors that he thought could influence tardigrade diversity and distribution. According to his findings, the type of bedrock and altitude seem to be the key determinants of the diversity and composition of tardigrade communities in Svalbard. Patterns resulting from the effects of altitude—usually negatively correlated with temperature—are of a particular interest as they may provide a reliable proxy for effects of climate change. Impacts of the current climate warming are expected to have particularly strong effects on the polar environments and their biota (e.g., Callaghan et al. 2004; Stempniewicz et al. 2007; Smykla et al. 2011). For example, in many climate change simulations Arctic species are at greater risk of severe range contraction and potential extinction than those in other climate zones (e.g., Parmesan 2006). Thus, obtaining data on diversity and distribution patterns of the Arctic biota is an important and urgent task.
Hornsund is located in west Spitsbergen, within the borders of the Sør-Spitsbergen National Park. Because of its diverse and pristine environments, it was chosen as one of the All Taxa Biodiversity Inventory (ATBI) sites (Kędra et al. 2010). Despite the abundance of studies focusing on marine environments surrounding Spitsbergen (for a summary see Kędra et al. 2010 and literature cited therein), only a few studies have explored the diversity and spatial patterns of terrestrial microbiota inhabiting this area (e.g., Węglarska 1965; Kaczmarek et al. 2012; Zmudczyńska et al. 2012; Ali et al. 2013; Zawierucha 2013).
This paper elucidates the biodiversity and distribution of the moss- and lichen-dwelling tardigrades in Hornsund. Given that samples were collected along two transects on the slopes of Ariekammen and Rotjesfjellet (Fig. 1), we were able to rigorously test the extent to which local environmental gradients, such as altitude and substrate type, determine tardigrade diversity and distribution in the Svalbard Archipelago.
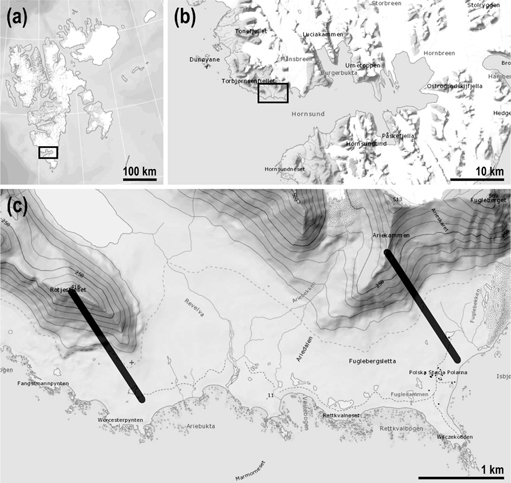
Fig. 1
The study area: (a) Svalbard Archipelago, (b) Hornsund fjord, west Spitsbergen, (c) surroundings of the Hornsund Station showing the location of the Rotjesfjellet (left) and Ariekammen (right) transects (maps from the Norwegian Polar Institute).
Materials and methods
Study area
The study area is located on the northern coast of Hornsund, Wedel Jarlsberg Land, west Spitsbergen (Fig. 1), in the vicinity of the Polish Polar Station Hornsund. The coastal landscape of the Hornsund area is dominated by mountain massifs with elevations exceeding 600 m a.s.l. and by coastal plains with several marine terraces covered with rich tundra vegetation. The massifs run parallel to the coast. The coastline of the fjord is very irregular, with several bays representing the lower reaches of valleys, which until recently have been occupied by retreating valley glaciers. The coastal ice-free areas also possess various topographical features characteristic for periglacial environments. Climate of this area is strongly affected by the ocean. However, climatic conditions are very variable and are characterized by low temperatures, low precipitation and strong foehn winds (Migała et al. 2008).
Vegetation in Hornsund is typical for Arctic tundra ecosystems. Specifically, it is dominated by various assemblages of low creeping dwarf shrubs, herbs, mosses and lichens. Variations of local environmental factors, which are related mainly to topography, geology, soil characteristics, influence of the ocean and glaciers, and to the presence of large colonies of seabirds determine development of particular plant assemblages (Stempniewicz et al. 2007). Mountain slopes facing the fjord host some of the world’s largest colonies of the little auk (Alle alle, Linnaeus, 1758). Seabirds contribute to the development of “ornithogenic tundra,” which is characterized by high primary production, rich plant cover and high diversity of plants and microbiota (e.g., Zmudczyńska et al. 2012; Ali et al. 2013). Both transects are characterized by lithosol (orthent) soils (Skiba 2013).
Field sampling
In August 2010, a field survey and sampling in the northern coast of the Hornsund area were carried out in order to investigate diversity of moss- and lichen-dwelling tardigrades. During this survey two transects were established and sampled along altitudinal gradients running on the slopes of Ariekammen (77°01′N; 15°31′E) and Rotjesfjellet (77°00′N; 15°22′E; Fig. 1). Both transects extended from the foothills to summits and samples were collected at ca. 30–50 m altitude intervals. At each altitude three to five moss, liverworts, lichen or mixed moss–lichen samples were collected from different substrates (i.e., rock or soil) and at least a few metres away from each other to ensure independent sampling. In total, 59 samples were collected on Ariekammen and Rotjesfjellet slopes (38 and 21, respectively). The list of all samples with their numbers, dry weights and associated geographic coordinates and altitudes is given in Supplementary Table S1.
Sample processing
Samples were collected and examined according to standard methods (e.g., Ramazzotti & Maucci 1983; Dastych 1985). All extracted specimens, exuvia and eggs were mounted on microscope slides in Hoyer’s medium. They were examined and then photographed using a BX 40 phase contrast microscope (Olympus, Shinjuku, Japan) associated with a ARTCAM-300MI camera (Artray Co., Tokyo, Japan) and QuickPhoto Camera 2.3 software. Species were identified using the key to the world Tardigrada (Ramazzotti & Maucci 1983) and later original descriptions, redescriptions and keys: Dastych (1985), Binda (1988), Bertolani & Rebecchi (1993), Tumanov (2006), Fontoura & Pilato (2007), Kaczmarek & Michalczyk (2009), Fontoura & Morais (2010), Kaczmarek, Gołdyn, Prokop et al. (2011) and Michalczyk et al. (2012a, b).
The measurements of Milnesium asiaticum Tumanov, 2006 males are given in micrometres (µm). Body length was measured from the mouth to the end of the body excluding the hind legs. Buccal tube length and level of the stylet support insertion point were measured from anterior margin of stylet sheaths. Buccal tube widths were measured as the external diameters at three levels of the buccal tube according Michalczyk et al. (2012a, b). Other measurements were made according to Tumanov (2006).
All samples and microscope slides are preserved in the Department of Animal Taxonomy and Ecology, Adam Mickiewicz University in Poznań, Poland.
Data analysis
In order to calculate species diversity, different indices and algorithms have been used. Alfa diversity (α) was expressed as the total number of species found in a particular sample. The overall species richness (regional or γ diversity) was obtained by pooling data from all samples. Moreover, the total number of species potentially present in the investigated area was estimated by two algorithms, Chao 2 and incidence-based coverage estimator (ICE). These indices estimate the overall number of species based on the observed number of species and the frequency of their occurrence in samples. In particular, the Chao 2 algorithm (Chao 1987) estimates the total number of species based on the number of species with one or two individuals only, whereas ICE is based on the number of species found in 10 or fewer samples (Lee & Chao 1994). Species accumulation curves and richness estimates (i.e., Chao 2 and ICE indices) were calculated using EstimateS 8.2 software (Colwell 2009). Because the computed value of Chao’s estimated coefficient of variation for abundance distribution was lower than 0.5, the Classic option was used instead of the bias-corrected option. Then, the larger value of the Chao 2 estimator was considered the best estimate of the abundance-based potential richness. In addition, a matrix of all pair-wise distances reflecting samples overlap in species composition was constructed.
As a measure of change in species composition between consecutive altitudinal zones, the turnover measure (β diversity) suggested by Wilson & Shmida (1984) was used. The turnover measure, based on pair-wise comparisons of the presence–absence data, was calculated with the following formula:
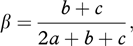
where a is the total number of species shared by the two adjacent altitudinal zones, b is the number of species present in the higher of the two zones but not in the lower one and c is the number of species present in the lower but not in the upper of the two zones in the comparison (Koleff et al. 2003).
Variation in species composition within samples and its relationship to environmental variables was investigated through the detrended canonical correspondence analysis (DCCA) using Canoco for Windows 4.5 software package (ter Braak & Šmilauer 2002). Based on weighted averaging of the environmental variables, this technique calculates species and environmental gradients and produces an ordination diagram that can be used to generate hypotheses about environmental determinants of species distribution. Since the number of samples was not equal in each sampling site, species data from individual samples were pooled within sampling sites, so the input species data matrix consisted of the total number of specimens for determined species in samples from individual sampling altitudes along the transects. Because in unimodal models (e.g., DCCA) rare species may have a disproportionately large influence on the calculations (ter Braak & Šmilauer 2002), species of which we found less than 20 specimens were excluded from the analysis. The matrix of attributable environmental data characterizing each sample consisted of host plant type (moss or lichen), substrate type (rock or soil) and altitude. Because we found tardigrades only in two mixed (moss–lichen) and in one liverwort sample, we excluded these samples from the analyses. Given that the transects do not differ in terms of soil type—all are lithosols (Skiba 2013)—and soil exposition, neither soil or slope were included in the model. The data incorporated the entire set of the recorded taxa with down-weighting of rare species by a default algorithm embedded in the Canoco for Windows 4.5 program (ter Braak & Šmilauer 2002). Significance of overall ordination was tested using Monte Carlo permutation tests (5000 permutations). To reduce the influence of spatial autocorrelation, permutations were restricted with regard to the spatial nature of the data; that is, blocks of data representing both transects (coded by a covariable) were permuted separately using cyclic shifts. The dry mass of the sample was used as a covariable in the analyses. Only species with the best fit to the model (>10% of fit) are presented on the DCCA graph. Analyses of covariance tested whether there were differences in species richness and abundance between the transects in relation to altitude. The total number of species or their abundance for each altitude in each transect was introduced to the model as the dependent variable, transect designation as the fixed factor and altitude and sample weight as covariables.
Results
Species composition and diversity
In samples collected from Ariekammen 924 specimens, 36 exuvia and 205 free-laid eggs were found with 814 specimens determined to the species level (Supplementary Table 2), whereas in samples collected on Rotjesfjellet 219 specimens, 18 exuvia and 43 free-laid eggs were found with 206 specimens determined to the species level (see also Kaczmarek et al. 2012). This makes a total of 1143 specimens found in moss, lichen, liverwort and mixed samples. The data set used for analyses contained 1020 specimens determined to 33 species. Of the 59 samples examined, 11 samples (seven from Rotjesfjellet and four from Ariekammen) did not contain any tardigrades. Data (i.e., site code, coordinates, sample numbers, sample dry weight, substratum, altitude) for all 59 samples are given in Supplementary Table S1. Species found in the study, together with their numbers and with taxonomic and zoogeographical remarks, are listed in Supplementary Table S2.
Overall (γ diversity), 33 species of Tardigrada belonging to 15 genera have been recorded along both transects. Of these, nine species representing four genera belong to Heterotardigrada and 24 species representing 11 genera belong to Eutardigrada. The estimated number of species based on all analysed samples was 52±17 for Chao 2 estimator and 41 for ICE.
Species richness for positive samples (α diversity) ranged from one to eight species. However, the majority of samples (64%) contained only one to four species (mean±SD=3.5±2.2). Differences in species composition between samples were considerable. None of the recorded species was found in all positive samples, and some species were found only in one (12 species) or two samples (three species). All these rare species were also represented mostly by only one or two, exceptionally more (but never more than 10) specimens. The most frequently recorded species occurring in samples were Echiniscus wendti Richters, 1903 (in 50% of positive samples), Macrobiotus islandicus islandicus Richters, 1904 (41%), M. h. obscurus Dastych, 1985 (38%), M. harmsworthi harmsworthi Murray, 1907 (24%) and M. hufelandi hufelandi C.A.S. Schultze, 1833 (15%). These species, together with Hypsibius pallidus Thulin, 1911 and Isohypsibius coulsoni Kaczmarek et al., 2012 (both found only in three samples), were also the most numerous and comprised 78% of the total number of extracted specimens. On the other hand, a remarkable number of species seem to be very rare in the investigated area. Over 50% of species (17 of 33) were represented by less than 1% of the total number of the collected specimens. Ten (30%) of the species found were represented only by one or two specimens (<0.2% of the total number of specimens).
Among six specimens of Milnesium asiaticum two males were identified (Fig. 2). This is the first ever record of males of this species. In general, the males were similar to females, but apart from having modified secondary branches of claws I (the diagnostic character of Milnesium males), they were also smaller than females. Measurements of their morphological structures are given in Supplementary Table S3.
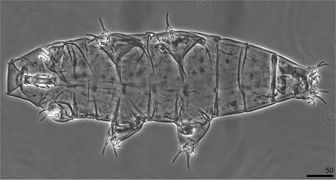
Fig. 2
One of the two males of Milnesium asiaticum Tumanov, 2006 found during the present study.
Species distribution along the altitudinal gradients
The first four DCCA ordination axes had eigenvalues of λI
=0.562, λII
=0.374, λIII
=0.048, and λIV
=0.033 and gradient lengths of 3.38, 3.21, 3.08 and 3.16 SD, respectively. The first and second eigenvalues are fairly high, whereas eigenvalues of consecutive axes are much weaker implying that only the first and the second axe represent fairly strong and meaningful environmental gradients. In fact, the first two axes taken together explained 90.4% of the species–environment relations and 37.1% of the variation in species distribution and abundance (sum of all canonical eigenvalues=1.272, total inertia=3.333). The species–environment correlations measuring the strength of the relationship between species and the environment for the first two axes were 0.89 and 0.90, respectively. Figure 3 shows the species and factor positions on the first two axes computed by DCCA. The Monte Carlo permutation test of the overall DCCA resulted in a significance of p=0.004 (F=3.056), and the model explained 38.2% of variation in species distribution and abundance.
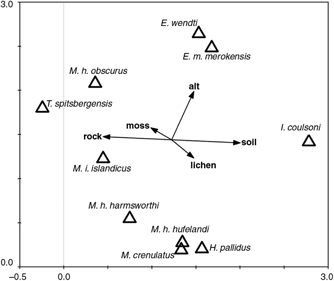
Fig. 3
Ordination diagram of detrended canonical correspondence analysis showing relation of particular tardigrade species (triangles) to altitude and types of substrate (moss or lichen, soil or rock; arrows). Eigenvalue of the first axis: 0.562; second axis: 0.374. Monte Carlo permutation test: F=3.446, p=0.004.
DCCA analysis (Fig. 3) indicated that altitude is the most important factor differentiating tardigrade communities in the investigated area. Macrobiotus crenulatus Richters, 1904, M. h. hufelandi and H. pallidus dominated in the lower elevations, whereas E. wendti, E. merokensis merokensis Richters, 1904 and M. h. obscurus prevailed in samples from the higher altitudes (Fig. 3). As many as 25% of moss samples were not inhabited by tardigrades. Among species found during the present study, I. coulsoni showed the strongest preference to the soil substratum. Still this species was more numerous in moss. In contrast, M. i. islandicus showed the strongest preference to the rock substratum. The proportion of lichen samples without tardigrades was 27%, and thus was very similar to that of the moss samples.
Analyses of covariance showed significant differences in species richness between the transects (F=9.125; p=0.008) although this variable was not significantly related to altitude (F=0.101; p=0.755). The model, including also the weight of the sample as a covariable, was marginally non-significant (F=3.233; p=0.051). Similarly, when total abundance of tardigrades was analysed in the same way, significant differences between the transects were observed (F=6.803; p=0.015), and there was no significant influence of altitude (F=0.160; p=0.694). The model, including also the weight of the sample as a covariable was not significant (F=2.477; p=0.099). Estimated average total number of determined specimens was 94 per 10 g of dry sample.
Species turnover (β diversity) between elevation zones did not show a uniform pattern. It increased along the altitudinal gradient up to 200 m a.s.l., it was very low between 200 and 250 m a.s.l., then it increased and was relatively constant up to 450 m a.s.l., and finally increased again at the highest elevations (Fig. 4). When comparing species occurring in the lowest elevation zone (14 m) with those found in the highest zone (500 m), three of seven species found in the low elevation were still present in the highest zone and four were missing compared with another 10 species occurring in the highest zone.
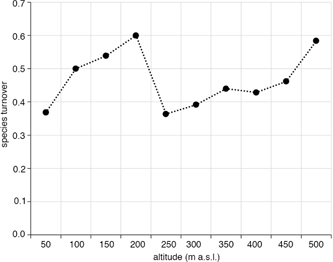
Fig. 4
Relationship between altitude and species turnover (β-diversity).
Discussion
Species richness and diversity
Taking into account the total number of 85 terrestrial and freshwater tardigrade species known from Spitsbergen (Zawierucha et al. 2013) and a relatively small area sampled during the present investigation, the number of 33 species (γ diversity) reported from Rotjesfjellet and Ariekammen could be considered high. However, a remarkable number of these species seem to be very rare in the investigated area. Half of the recorded species constitute less than 1% of the total number of the collected specimens. Rare species are generally likely to be missed in sampling campaigns, but very high small-scale spatial variability and patchy distribution of tardigrades makes it likely that even in well-studied areas many species can be easily overlooked (e.g., Meyer 2006b; Meyer & Hinton 2007; Degma et al. 2010). Based on the Chao 2 and ICE indices, the total number of tardigrade species present in the investigated area may range between 35 and 69 species, a 6–109% increase compared to the observed number. Although ICE is known to overestimate species richness when many of the recorded species are rare (Kaya et al. 2010), the high number of possible omitted species was also supported by the Chao 2 index, which in fact produced estimates higher than the ICE index. Chao 2 index in the Hornsund area indicates that the currently reported biodiversity of Arctic tardigrades is underestimated.
Data from previous surveys also indicate a much higher diversity of tardigrades inhabiting this area. Terrestrial tardigrades of the northern coast of Hornsund were investigated by Węglarska (1965) and Dastych (1985). They did not investigate Rotjesfjellet, but other slopes of Ariekammen from which they reported five species that were not found during the present investigation, that is, Ramazzottius oberhaeuseri (Doyère, 1840), Tenuibiotus willardi (Pilato, 1977), Milnesium tardigradum Doyère, 1840 sensu lato, Macrobiotus ariekammensis Węglarska, 1965 and Macrobiotus echinogenitus Richters, 1904. Moreover, a relatively high number of species new to Spitsbergen found in other samplings (see Kaczmarek et al. 2012; Zawierucha 2013) indicates that diversity of the tardigrade fauna of the Hornsund area is probably considerably underestimated. The high tardigrade biodiversity of the area and the data from other High Arctic localities (e.g., Pilato et al. 2006; Johansson et al. 2013; Vár Trygvadóttir & Kristensen 2013) show that these extreme environments can be colonized by high numbers of tardigrade species.
Approximately 80% of samples examined in the present investigation contained tardigrades. This is in accordance with the majority of previous studies which usually reported between 70 to over 90% of samples containing tardigrades and/or their eggs (e.g., Hallas 1978; Dastych 1985; Jönsson 2003; Guil, Sánchez-Moreno et al. 2009). Species richness per positive sample (α diversity) recorded in the present study (one to eight species) is also consistent with previous observations of Spitsbergen tardigrade diversity; for example, Dastych (1985) reported one to 10 species per sample. Tardigrade communities usually exhibit low α diversity and the number of tardigrade species per cryptogam sample usually ranges from two to six (e.g., Ramazzotti & Maucci 1983; Meyer 2006a).
Altitude and tardigrade distribution
The DCCA analysis showed that altitude seems to be the most important factor shaping tardigrade communities in the Hornsund. A significant relationship between the structure of tardigrade communities and elevation has been documented by a number of studies (e.g., Rodriguez-Roda 1951; Nelson 1975; Dastych 1980, 1985, 1987, 1988; Beasley 1988; Collins & Bateman 2001; Guil, Hortal et al. 2009; Kaczmarek, Gołdyn, Wełnicz et al. 2011). However, it is not clear which variables covarying with elevation (e.g., climate, vegetation or others) are responsible for shaping tardigrade diversity, distribution and abundance.
To investigate potential impacts of climatic changes along studied altitude gradients, we selected well exposed and relatively mildly rising transects with homogeneous physiognomy, similar habitats and vegetation structure. To eliminate potential effects of slope exposure to sunlight (Nelson 1975; Nichols et al. 2001), both transects were established on south-eastern facing slopes. Assuming a linear relationship between altitude and air temperature of 0.83°C per 100 m of elevation (Migała et al. 2008), the investigated altitudinal gradient, spanning approximately from sea level to over 500 m a.s.l., represents a temperature gradient of about 4.3°C. Such differences in mean temperatures are consistent with the range in temperature change predicted by general climatic models, which indicate that by the end of this century average air temperatures in most of the High Arctic areas may increase by ca. 5°C (Symon et al. 2005). The investigated altitudinal transects may be useful as a proxy for predicting climate change effects on tardigrade biodiversity. As hypothesized by Vicente (2010), an increase in temperatures may negatively affect exclusively limnic tardigrades which are not capable of cryptobiosis (Rebecchi et al. 2009).
At each elevation zone, we collected samples of mosses and lichens dominant at these elevations; thus, the investigated tardigrade assemblages came from different moss and lichen species. Although it has been suggested that substrate species may influence tardigrade community composition (e.g., Dastych 1980, 1985, 1988; Hofmann 1987), and the issue has been widely debated in literature, so far there is no convincing evidence for any specific associations between moss species and tardigrade distribution and abundance (e.g., Nelson 1975; Ramazzotti & Maucci 1983; Kathman & Cross 1991; Meyer 2006a). It has also been found that the same moss species growing in different environmental conditions contained different tardigrade communities (Jönsson 2003; Guil, Hortal et al. 2009; Kaczmarek, Gołdyn, Wełnicz et al. 2011). Taking into account that a given cryptogam species may appear in a range of environmental conditions, it seems unlikely that the plant species itself determines the occurrence and diversity of tardigrades. The growth form of a moss or lichen, and its effect on micro-environmental conditions (i.e., moisture), is probably more significant than the substrate species (e.g., Jönsson 2003, 2007; Meyer 2006a; Guil, Hortal et al. 2009; Guil, Sánchez-Moreno et al. 2009). Still, the overall meso-climatic regimes of the environment in which the cryptogam grows (such as rainfall, humidity, exposure, insulation and temperature) seem to be chiefly responsible for the development and maintenance of tardigrade populations (Schuster & Greven 2007; Guil, Hortal et al. 2009; Guil, Sánchez-Moreno et al. 2009; Kaczmarek, Gołdyn, Wełnicz et al. 2011). It therefore seems reasonable to conclude that neither the type of vegetation nor differences in moss and lichen species significantly influenced tardigrade composition along the altitudinal gradients investigated in the present study.
Consistent with our findings, Dastych (1985) reported a negative relationship between tardigrade species richness and altitude in samples collected in Spitsbergen. Other studies provide conflicting results, reporting a positive (e.g., Rodriguez-Roda 1951; Nelson 1975; Dastych 1987, 1988; Kaczmarek, Gołdyn, Wełnicz et al. 2011), a negative (e.g., Dastych 1980; Beasley 1988; Collins & Bateman 2001) or no relationships (e.g., Kathman & Cross 1991; Nichols et al. 2001) between tardigrade species richness and elevation. However, this inconsistency may be illusory and results reported by different authors may in fact be complementary. As indicated by Guil, Hortal et al. (2009), tardigrade species richness shows a unimodal distribution along an altitudinal gradient. Although some authors (e.g., Rodriguez-Roda 1951; Dastych 1987) reported that the number of species increases with altitude, a closer examination of their data in fact indicates a unimodal distribution of tardigrade richness. In other words, non-linear functions should also be fitted to data in order to test whether they explain a greater amount of variance than a linear function. Moreover, all three possible relationships may be identified depending on the altitude range investigated. Specifically, if the relationship over large ranges is indeed unimodal, then when analysing smaller ranges encompassing lower altitudes one may find a positive relationship, at intermediate altitudes no relationship, and finally at higher altitudes a negative relationship between altitude and species richness.
As indicated above, Dastych (1985) found a continuous decreasing trend in tardigrade species richness from the lowest elevation zones to the upper parts of the highest summits. However, his results were probably heavily affected by unbalanced sample sizes resulting from a significantly lower (5–10 times) number of samples collected from the highest compared to the lowest elevation zones. Very high levels of small-scale spatial variability in tardigrade distribution (Meyer 2006b) make it likely that rare species have been missed in higher, less sampled elevation zones.
Similarly to species richness, there is also some controversy about the relationships of tardigrade abundance with altitude. Some previous studies have reported an increase (e.g., Dastych 1980, 1987, 1988), a decrease (e.g., Dastych 1985; Beasley 1988; Collins & Bateman 2001) or no changes (e.g., Kathman & Cross 1991; Nichols et al. 2001; Guil et al. 2009; Johansson et al. 2013) in tardigrade abundance with increasing altitude. Although Dastych (1985) reported that in Svalbard tardigrade abundance decreases distinctly with elevation, closer examination of his data indicate that tardigrade abundance shows a unimodal relationship with altitude, reaching the highest values between 100 and 300 m a.s.l. Our study, however, did not detect an effect of altitude on tardigrade abundance. The proportion of positive samples also did not change with altitude. In this respect, the present results correspond well with previous investigations conducted on Spitsbergen by Dastych (1985). However, other studies conducted in the tropics (Kaczmarek, Gołdyn, Wełnicz et al. 2011) and in temperate (Dastych 1988) climatic zones showed that the proportion of positive samples may vary between 0 and 100% and it is positively correlated with altitude, with 0–50% positives in lowlands and 50–100% positives in highlands.
Although not directly investigated, our data provide some insight into the question of relations between chemical properties of the environment and tardigrade communities. In our study (Fig. 4), the species turnover (β diversity) generally increased along the altitudinal gradients. At lower elevations, the increase was mostly related to gained species. However, at 200 m a.s.l. both the fraction of gained and lost species was high, resulting in very high species turnover rates. The species turnover and composition were relatively constant up to the highest elevation zone, where the proportion of gained and lost species was high again. This pattern suggests abrupt changes in environmental conditions influencing tardigrade communities around 250 and above 450 m a.s.l., with relatively constant climate conditions between these elevations. This elevation range roughly corresponds with the distribution of little auk colonies on the slopes of Ariekammen and Rotjesfjellet. During their reproductive season, these seabirds deposit enormous amounts of guano, which is known to provide nutrients for poor tundra ecosystems and to considerably affect local biogeochemistry (Stempniewicz et al. 2007). Recent studies conducted on slopes of Ariekammen showed that the nutrients positively affected soil collembolan communities (Zmudczyńska et al. 2012). Also, studies carried out in the Antarctic indicate a high impact of seabird colonies on soil metazoans, including tardigrades (Porazińska et al. 2002; Smykla et al. 2010). Zawierucha, Cytan et al. (2015) have shown that A. alle guano positively influences tardigrade body size. Transects sampled during the present study did not run through the colonies, but the impact of colonial birds was shown to affect large areas of tundra around the colonies (Smykla et al. 2007; Stempniewicz et al. 2007). Therefore, despite our attempts to keep constant as many variables as possible, it is probable that abrupt shifts in species composition recorded at heights 250 and 450 m a.s.l. were related to the nearby seabird colonies. The putative influence of guano on the composition and abundance of tardigrade communities certainly requires further investigation.
Remarks on males of Milnesium asiaticum Tumanov, 2007
Up to now, M. asiaticum has been recorded only three times, by Tumanov (2007), by Kaczmarek et al. (2012) and by Zawierucha et al. (2014), but males have not been found at any of these occasions. In fact, a majority of limno-terrestrial tardigrade species are thought to be parthenogenetic (Bertolani 2001). Moreover, the type of reproduction may vary not only between different tardigrade species but also between populations of the same species (Nelson et al. 2010; Lemloh et al. 2011).
Acknowledgements
The material for this work was collected during the 33rd Polar Expedition of the Polish Academy of Sciences to Svalbard in 2010. We thank the Department of Polar and Marine Research Institute of Geophysics of the Polish Academy of Sciences for providing logistical support during the fieldwork, and the team of the Stanislaw Siedlecki Polish Polar Station in Hornsund for their hospitality and assistance. Field survey and sampling was conducted within Research in Svalbard project no. 5326 and was financially supported by National Science Centre grant no. NN305376438 to JS. Funding for this work was also provided by the programme Supporting International Mobility of Scientists, edition III, project no. 2, to JS, by the Polish Ministry of Science and Higher Education via the Diamond Grant scheme grant no. DI2011 035241 to KZ and by the National Science Centre grant no. NN304014939 to ŁK, ŁM and JS. KZ is a beneficiary of the Adam Mickiewicz Foundation scholarship scheme.
References
Ali
S.H.,
Alias
S.A.,
Siang
H.Y.,
Smykla
J.,
Pang
K.L.,
Guo
S.Y.
&
Convey
P. 2013.
Studies on diversity of soil microfungi in the Hornsund area, Spitsbergen. Polish Polar Research 34,
39–54.
Beasley
C.W. 1988.
Altitudinal distribution of Tardigrada of New Mexico with the description of a new species. The American Midland Naturalist 120,
436–440.
Publisher Full Text
Bertolani
R. 2001.
Evolution of the reproductive mechanisms in tardigrades—a review. Zoologisher Anzeiger 240,
247–252.
Publisher Full Text
Bertolani
R.,
Guidetti
R.,
Jönsson
K.I.,
Altiero
T.,
Boschini
D.
&
Rebecchi
L. 2004.
Experiences on dormancy in tardigrades. Journal of Limnology 63,
16–25.
Publisher Full Text
Bertolani
R.
&
Rebecchi
L. 1993.
A revision of the Macrobiotus hufelandi group (Tardigrada, Macrobiotidae), with some observations on the taxonomic characters of eutardigrades. Zoologica Scripta 22,
127–152.
Publisher Full Text
Binda
M.G. 1988.
Redescrizione di Macrobiotus echinogenitus Richters, 1904 e sul valore di buona specie di Macrobiotus crenulatus Richters, 1904 (Eutardigrada). (Rediscription of Macrobiotus echinogenitus Richters, 1904 and Macrobiotus crenulatus Richters, 1904 bona species [Eutardigrada].) Animalia 15,
201–210.
Callaghan
T.V.,
Björn
L.O.,
Chernov
Y.,
Chapin
T.,
Christensen
T.R.,
Huntley
B.,
Ims
R.A.,
Johansson
M.,
Jolly
D.,
Jonasson
S.,
Matveyeva
N.,
Panikov
N.,
Oechel
W.,
Shaver
G.
&
Henttonen
H. 2004.
Effects on the structure of Arctic ecosystems in the short- and long-term perspectives. Ambio 33,
436–447.
PubMed Abstract
Chao
A. 1987.
Estimating the population size for capture–recapture data with unequal catchability. Biometrics 43,
783–791.
PubMed Abstract | Publisher Full Text
Collins
M.
&
Bateman
L. 2001.
The ecological distribution of tardigrades in Newfoundland. Zoologischer Anzeiger 240,
291–297.
Publisher Full Text
Colwell
R.K. 2009. EstimateS. Statistical estimation of species richness and shared species from samples. Version 8.2.
Downloaded from the internet at http://viceroy.eeb.uconn.edu/estimates in 2012.
Convey
P.
&
McInnes
S.J. 2005.
Exceptional, tardigrade dominated ecosystems in Ellsworth Land, Antarctica. Ecology 86,
519–527.
Publisher Full Text
Dastych
H. 1980. Niesporczaki (Tardigrada) Tatrzańskiego Parku Narodowego. (Water bears [Tardigrada] of Tatrzański National Park.) Monografie Fauny Polski 9.
Warsaw: Państwowe Wydawnictwo Naukowe.
Dastych
H. 1985.
West Spitsbergen Tardigrada. Acta Zoologica Cracviensia 28,
169–214.
Dastych
H. 1987.
Altitudinal distribution of Tardigrada in Poland.
In R. Bertolani (ed.): Biology of tardigrades. Selected Symposia and Monographs U. Z. I. Vol. 1. Pp. 169–176. Modena: Mucchi Editore.
Dastych
H. 1988. The Tardigrada of Poland. Monografie Fauny Polski 16.
Warsaw: Państwowe Wydawnictwo Naukowe.
Degma
P.,
Bertolani
R.
&
Guidetti
R. 2011. Actual checklist of Tardigrada species. Accessed on the internet at http://www.tardigrada.modena.unimo.it/miscellanea/Actual checklist of Tardigrada.pdf on 31 October 2014.
Degma
P.,
Katina
S.
&
Sabatovičová
L. 2010.
Horizontal distribution of moisture and Tardigrada in a single moss cushion. Journal of Zoological Systematics and Evolutionary Research 49, Suppl. 1,
71–77.
Fontoura
P.
&
Morais
P. 2010.
Assessment of traditional and geometric morphometrics for discriminating cryptic species of the Pseudechiniscus suillus complex (Tardigrada, Echiniscidae). Journal of Zoological Systematics and Evolutionary Research 49(Suppl. 1),
26–33.
Fontoura
P.
&
Pilato
G. 2007. Diphascon (Diphascon) faialense sp. nov. a new species of Tardigrada (Eutardigrada, Hypsibiidae) from Azores and a key to the species of the D. pingue group. Zootaxa 1589,
47–55.
Garey
J.R.,
McInnes
S.J.
&
Nichols
P.B. 2008.
Global diversity of tardigrades (Tardigrada) in freshwater. Hydrobiologia 595,
101–106.
Publisher Full Text
Guidetti
R.,
Altiero
T.,
Bertolani
R.,
Grazioso
P.
&
Rebecchi
L. 2011.
Survival of freezing by hydrated tardigrades inhabiting terrestrial and freshwater habitats. Zoology (Jena) 114,
123–128.
PubMed Abstract | Publisher Full Text
Guil
N. 2008.
New records and within-species variability of Iberian tardigrades (Tardigrada), with comments on the species from the Echiniscus-blumi-canadensis series. Zootaxa 1757,
1–30.
Guil
N.
&
Cabrero-Sanudo
F.J. 2007.
Analysis of the species description process of a little known invertebrate group: the limnoterrestrial tardigrades (Bilateria, Tardigrada). Biodiversity and Conservation 16,
1063–1086.
Publisher Full Text
Guil
N.,
Hortal
J.,
Sánchez-Moreno
S.
&
Machordom
A. 2009.
Effects of macro and micro-environmental factors on the species richness of terrestrial tardigrade assemblages in an Iberian mountain environment. Landscape Ecology 24,
375–390.
Publisher Full Text
Guil
N.,
Sánchez-Moreno
S.
&
Machordom
A. 2009.
Local biodiversity patterns in micrometazoans: are tardigrades everywhere? Systematics and Biodiversity 7,
259–268.
Publisher Full Text
Hallas
T.E. 1978.
Habitat preference in terrestrial tardigrades. Annales Zoologici Fennici 15,
66–68.
Hofmann
I. 1987.
Habitat preference of the most frequent moss-living Tardigrada in the area of Giessen (Hessen).
In R. Bertolani (ed.): Biology of tardigrades. Selected Symposia and Monographs U.Z.I. Vol. 1. Pp. 211–216. Modena: Mucchi Editore.
Johansson
C.,
Miller
W.R.,
Linder
E.T.,
Adams
B.J.
&
Boreliz-Alvarado
E. 2013.
Tardigrades of Alaska: distribution patterns, diversity and species richness. Polar Research 32,
1–11.
Publisher Full Text
Jönsson
K.I. 2003.
Population density and species composition of moss-living tardigrades in a boreo-nemoral forest. Ecography 26,
356–364.
Publisher Full Text
Jönsson
K.I. 2007. Long-term experimental manipulation of moisture conditions and its impact on moss-living tardigrades. Journal of Limnology, 66, Suppl. 1, 119–125.
Kaczmarek
Ł.,
Gołdyn
B.,
Prokop
Z.M.
&
Michalczyk
Ł. 2011.
New records of Tardigrada from Bulgaria with the description of Macrobiotus binieki sp. nov. (Eutardigrada: Macrobiotidae) and a key to the species of the harmsworthi group. Zootaxa 2781,
29–39.
Kaczmarek
Ł.,
Gołdyn
B.,
Wełnicz
W.
&
Michalczyk
Ł. 2011.
Ecological factors determining Tardigrada distribution in Costa Rica. Journal of Zoological Systematics and Evolutionary Research 49,
78–83.
Publisher Full Text
Kaczmarek
Ł.
&
Michalczyk
Ł. 2009.
Redescription of Hypsibius microps Thulin, 1928 and H. pallidus Thulin, 1911 (Eutardigrada: Hypsibiidae) based on the type material from the Thulin collection. Zootaxa 2275,
60–68.
Kaczmarek
Ł.,
Zawierucha
K.,
Smykla
J.
&
Michalczyk
Ł. 2012.
Tardigrada of the Revdalen (Spitsbergen) with the descriptions of two new species: Bryodelphax parvuspolaris (Heterotardigrada) and Isohypsibius coulsoni (Eutardigrada). Polar Biology 35,
1013–1026.
Publisher Full Text
Kathman
R.D.
&
Cross
S.F. 1991.
Ecological distribution of moss-dwelling tardigrades on Vancouver Island, British Columbia, Canada. Canadian Journal of Zoology 69,
122–129.
Publisher Full Text
Kaya
M.,
De Smet
W.H.
&
Fontaneto
D. 2010.
Survey of moss-dwelling bdelloid rotifers from middle Arctic Spitsbergen (Svalbard). Polar Biology 33,
833–842.
Publisher Full Text
Kędra
M.,
Gromisz
S.,
Jaskuła
R.,
Legeżyńska
J.,
Maciejewska
B.,
Malec
E.,
Opaniowski
A.,
Ostrowska
B.,
Włodarska-Kowalczuk
M.
&
Węsławski
J.M. 2010.
Soft bottom macrofauna of an All Taxa Biodiversity Site: Hornsund (77°N, Svalbard). Polish Polar Research 31,
309–326.
Koleff
P.,
Gaston
K.
&
Lennon
J.L. 2003.
Measuring beta diversity for presence-absence data. Journal of Animal Ecology 72,
367–382.
Publisher Full Text
Lee
S.-M.
&
Chao
A. 1994.
Estimating population size via sample coverage for closed capture–recapture models. Biometrics 50,
88–97.
PubMed Abstract | Publisher Full Text
Lemloh
M.-L.,
Brümmer
F.
&
Schill
R.O. 2011.
Life-history traits of the bisexual tardigrades Paramacrobiotus tonollii and Macrobiotus sapiens. Journal of Zoological Systematics and Evolutionary Research 49,
58–61.
Publisher Full Text
McInnes
S.J.
&
Pugh
P.J.A. 2007.
An attempt to revisit the global biogeography of limno-terrestrial Tardigrada. Journal of Limnology 66,
90–96.
Publisher Full Text
Meyer
H.A. 2006a.
Intraspecific association and substrate specificity in tardigrades from Florida, southeastern United States. Hydrobiologia 558,
129–132.
Publisher Full Text
Meyer
H.A. 2006b.
Small-scale spatial distribution variability in terrestrial tardigrade populations. Hydrobiologia 558,
133–139.
Publisher Full Text
Meyer
H.A.
&
Hinton
J.G. 2007.
Limno-terrestrial Tardigrada of the Nearctic realm. Journal of Limnology 66,
97–103.
Publisher Full Text
Michalczyk
Ł.,
Wełnicz
W.,
Frohme
M.
&
Kaczmarek
Ł. 2012a.
Redescriptions of three Milnesium Doyère, 1840 taxa (Tardigrada: Eutardigrada: Milnesiidae), including the nominal species for the genus. Zootaxa 3154,
1–20.
Michalczyk
Ł.,
Wełnicz
W.,
Frohme
M.
&
Kaczmarek
Ł. 2012b.
Corrigenda of Zootaxa, 3154: 1–20. Redescriptions of three Milnesium Doyère, 1840 taxa (Tardigrada: Eutardigrada: Milnesiidae), including the nominal species for the genus. Zootaxa 3393,
66–68.
Migała
K.,
Nasiółkowski
T.
&
Pereyma
J. 2008.
Topoclimatic conditions in the Hornsund area (SW Spitsbergen) during the ablation season 2005. Polish Polar Research 29,
73–91.
Nelson
D.R. 1975.
Ecological distribution of tardigrades on Roan Mountain, Tennessee—North Carolina. Memorie dell’Istituto Italiano di Idrobiologia 32,
225–276.
Nelson
D.R.,
Guidetti
R.
&
Rebecchi
L. 2010.
Tardigrada.
In J.H. Torp & A. Covich (eds.): Ecology and classification of North American freshwater invertebrates. Pp. 527–550. London: Academic Press.
Nichols
P.B.,
Romano
F.A.
&
Nelson
D.R. 2001.
Seasonal and altitudinal variation in the distribution and abundance of Tardigrada on Dugger Mountain, Alabama. Zoologischer Anzeiger 240,
501–504.
Publisher Full Text
Parmesan
C. 2006.
Ecological and evolutionary responses to recent climate change. Annual Review of Ecology, Evolution, and Systematics 37,
637–669.
Publisher Full Text
Pilato
G.,
Guidetti
R.,
Rebecchi
L.,
Lisi
O.,
Hansen
J.G.
&
Bertolani
R. 2006.
Geonemy, ecology, reproductive biology and morphology of the tardigrade Hypsibius zetlandicus (Eutardigrada: Hypsibiidae) with erection of Borealibius gen. n. Polar Biology 29,
595–603.
Publisher Full Text
Porazińska
D.L.,
Wall
D.H.
&
Wirginia
R.A. 2002.
Invertebrates in ornithogenic soils on Ross Island, Antarctica. Polar Biology 25,
569–574.
Ramazzotti
G.
&
Maucci
W. 1983. II Phylum Tardigrada. III. edizione riveduta e aggiornata. (II Phylum Tardigrada. 3rd edited and updated edition.) Memorie dell’Istituto Italiano di Idrobiologia 41.
Pallanza: Italian Institute of Hydrobiology.
Rebecchi
L.,
Boschini
D.,
Cesari
M.,
Lencioni
V.,
Bertolani
R.
&
Guidetti
R. 2009.
Stress response of a boreo-alpine species of tardigrade, Borealibilis zetlandicus (Eutardigrada, Hypsibiidae). Journal Limnology 81,
64–70.
Publisher Full Text
Rodriguez-Roda
J. 1951.
Algunos datos sobre la distribucion de los tardigrados espanoles. (Some data on distribution of Spanish tardigrades.) Boletin de la Real Sociedad Espanola de Historia Natural. Seccion Biologica 49,
75–83.
Schuster
R.
&
Greven
H. 2007.
A long term study of population dynamics of tardigrades in the moss Rhytidiadelphus squarrosus (Hedw.) Warnst. Journal of Limnology 66,
141–151.
Publisher Full Text
Scourfield
D.J.
1897. Contributions to the non-marine fauna of Spitsbergen. Part I. Preliminary notes, and reports on the Rhizopoda, Tardigrada, Entomostraca, etc. Proceedings of the Zoological Society of London 65(3),
784–792.
Skiba
S. 2013.
Geographical environment in the vicinity of the Stanisław Siedlecki Polish Polar Station in Hornsund. Soils.
In Z. Zwoliński et al. (eds.): Ancient and modern geoecosystems of Spitsbergen. Pp. 57–100. Poznań: Bogucki Wydawnictwo Naukowe.
Smykla
J.,
Kaczmarek
Ł.,
Huzarska
K.
&
Michalczyk
Ł. 2011.
The first record of a rare marine tardigrade, Halobiotus crispae Kristensen, 1982 (Eutardigrada: Hypsibiidae), from the Svalbard Archipelago. Polar Biology 34,
1243–1247.
Publisher Full Text
Smykla
J.,
Porazińska
D.L.,
Iakovenko
N.,
Janko
K.,
Weiner
W.M.,
Niedbała
W.
&
Drewnik
M. 2010.
Studies on the Antarctic soil invertebrates: preliminary data on rotifers (Rotatoria) with notes on other taxa from Edmonson Point (northern Victoria Land, continental Antarctic). Acta Societatis Zoologicae Bohemicae 74,
135–140.
Smykla
J.,
Wołek
J.
&
Barcikowski
A. 2007.
Zonation of vegetation related to penguin rookeries on King George Island, Maritime Antarctic. Arctic, Antarctic and Alpine Research 39,
143–151.
Sohlenius
B.
&
Boström
S. 2008.
Species diversity and random distribution of microfauna in extremely isolated habitable patches on Antarctic nunataks. Polar Biology 31,
817–825.
Publisher Full Text
Stempniewicz
L.,
Błachowiak-Samołyk
K.
&
Węsławski
J.M. 2007.
Impact of climate change on zooplankton communities, seabird populations and Arctic terrestrial ecosystem—a scenario. Deep-Sea Research Part II 54,
2934–2945.
Publisher Full Text
Symon
C.,
Arris
L.
&
Heal
B. 2005. Arctic climate impact assessment. New York: Cambridge University Press.
ter Braak
C.J.F.
&
Šmilauer
P. 2002. CANOCO reference manual and CanoDraw for Windows user’s guide: software for Canonical Community Ordination (version 4.5).
Ithaca,
NY: Microcomputer Power.
Tumanov
D.V. 2006.
Five new species of the genus Milnesium (Tardigrada, Eutardigrada, Milnesiidae). Zootaxa 1122,
1–23.
Vár Trygvadóttir
B.
&
Kristensen
R.M. 2013.
A zoogeographic study of the limnoterrestrial tardigrade fauna on the Faroe Islands. Journal of Limnology 72,
113–122.
Vicente
F. 2010.
Micro-invertebrates conservation: forgotten biodiversity. Biodiversity and Conservation 19,
3629–3634.
Publisher Full Text
Vicente
F.
&
Bertolani
R. 2013.
Considerations on the taxonomy of the Phylum Tardigrada. Zootaxa 3626,
245–248.
Publisher Full Text
Węglarska
B. 1965.
Die Tardigraden (Tardigrada) Spitzbergens. (The tardigrades [Tardigrada] of Spitsbergen.) Acta Zoologica Cracoviensia 11,
43–51.
Wełnicz
W.,
Grohme
M.A.,
Kaczmarek
Ł.,
Schill
R.O.
&
Frohme
M. 2011.
Anhydrobiosis in tardigrades—the last decade. Journal of Insect Physiology 57,
577–583.
Publisher Full Text
Wilson
M.V.
&
Shmida
A. 1984.
Measuring beta-diversity with presence–absence data. Journal of Ecology 72,
1055–1064.
Publisher Full Text
Zawierucha
K. 2013.
Some Tardigrada from Arctic tundra (Svalbard, Spitsbergen) with a description of Isohypsibius karenae (Eutardigrada: Isohypsibiidae). Polish Polar Research 34,
383–396.
Publisher Full Text
Zawierucha
K.,
Coulson
S.,
Michalczyk
Ł.
&
Kaczmarek
Ł.
2013. Current knowledge of the Tardigrada of Svalbard with the first records of water bears from Nordaustlandet (High Arctic). Polar Research
32, article no. 20886, doi: 10.3402/polar.v32i0.20886.
Publisher Full Text
Zawierucha
K.,
Cytan
J.,
Smykla
J.,
Wojczulanis-Jakubas
K.,
Kaczmarek
Ł.,
Kosicki
J.Z.
&
Michalczyk
Ł.
2015. Sea bird guano boosts body size of water bears (Tardigrada) inhabiting the Arctic tundra. Polar Biology. doi: 10.1007/s00300-014-1591-x.
Publisher Full Text
Zawierucha
K.,
Dziamięcki
J.,
Jakubowska
N.,
Michalczyk
Ł.
&
Kaczmarek
Ł. 2014.
New tardigrade records for the Baltic states with a description of Minibiotus formosus sp. nov. (Eutardigrada, Macrobiotidae). Zookeys 408,
81–105.
PubMed Abstract | Publisher Full Text
Zmudczyńska
K.,
Olejniczak
I.,
Zwolicki
A.,
Iliszko
L.,
Convey
P.
&
Stempniewicz
L. 2012.
Influence of allochtonous nutrients delivered by colonial seabirds on soil collembolan communities on Spitsbergen. Polar Biology 35,
1233–1245.
Publisher Full Text