RESEARCH/REVIEW ARTICLE
Adjustment of pigment composition in Desmarestia (Desmarestiaceae) species along a sub-Antarctic to Antarctic latitudinal gradient
Andrés Mansilla,1,2 Fabio Méndez,1,2,3 Silvia Murcia,1 Juan Pablo Rodríguez,1,2,3 Johanna Marambio,1,2,3 Sebastián Rosenfeld,1,2,3 Nair Yokoya4 & Kai Bischof5
1 Laboratory of Antarctic and Sub-Antarctic Marine Ecosystems, University of Magallanes, 01855 Bulnes Ave., Punta Arenas 6200000, Chile
2 Institute of Ecology and Biodiversity, Las Palmeras St. 3425, Ñuñoa, Santiago 8320000, Chile
3 Conservation and Management of Natural Resources in Sub-Antarctic Environments MS Program, University of Magallanes, 01855 Bulnes Ave., Punta Arenas 6200000, Chile
4 Institute of Botany, São Paulo State Department of the Environment, 3687 Miguel Estéfano Ave., São Paulo 04301-012, SP, Brazil
5 Marine Botany Department, University of Bremen, NW2 Leobener St., Bremen DE-28359, Germany
Abstract
Photosynthesis at high latitudes demands efficient strategies of light utilization to maintain algal fitness and performance. The fitness, and physiological adaptation, of a plant or algae species depends in part on the abundance and efficiency of the pigments it can produce to utilize the light resource from its environment. We quantified pigment composition and concentration in six species of the brown macroalgal genus Desmarestia, collected from sub-Antarctic sites (Strait of Magellan, Beagle Channel–Cape Horn Province) and sites on the Antarctic Peninsula and adjacent islands. Sub-Antarctic Desmarestia species exhibited lower concentrations of chlorophyll a, chlorophyll c and fucoxanthin than endemic Antarctic species. Antarctic samples of D. menziesii and D. antarctica collected along a decreasing latitudinal gradient showed spatial and interspecific differences in light-harvesting pigment composition. Our results suggest distinct physiological adjustments in Desmarestia species in response to heterogeneous abiotic environmental conditions. The marine sub-Antarctic and Antarctic ecosystems are characterized by harsh environments (e.g., extreme irradiance, photoperiod, temperature, salinity) to which the physiology of macroalgal species must adapt.
Keywords
Macroalgae; Phaeophyceae; photosynthesis; physiology; environmental heterogeneity; Chile.
Citation: Polar Research 2016, 35, 29383, http://dx.doi.org/10.3402/polar.v35.29383
Copyright: © 2016 A. Mansilla et al. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License, permitting all non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Published: 4 August 2016
Correspondence to: Silvia Murcia, Laboratory of Antarctic and Sub-Antarctic Marine Ecosystems, University of Magallanes, 01855 Bulnes Ave., Punta Arenas 6200000, Chile. E-mail: silvia.murcia@umag.cl
The Chilean sub-Antarctic ecoregion of Magallanes (or Magellan) hosts a coastal, benthic community that is highly distinct from other temperate rocky shores on the South American Continent in terms of species composition, richness and structure. Such distinctions could be the result of several factors, such as the geomorphology generated by the glacial erosion during the advance and retreat of ice in the Quaternary (Silva & Calvete 2002); oceanographic gradients combining unique current flows, salinity, and thermal (Dayton 1985; Silva & Calvete 2002), photoperiod and irradiance regimes; presence of glaciers with predominant west-to-east gradients of winds and rainfall (Aravena & Luckman 2009) and freshwater coastal discharge; variable substrate types with abrupt change in geomorphology of the sub-Antarctic system of fjords and channels (Valdenegro & Silva 2003), resulting in unique physical and biogeochemical seawater conditions (e.g., nutrient cycles, carbonate system dynamics [Torres et al. 2014]) that generate a distinct algal structure. The environmental heterogeneity governing sub-Antarctic and Antarctic coastal ecosystems has shaped the evolutionary history and physiological adaptations of the local macroalgal flora.
From phylogenetic, morphological and eco-physiological studies of the genus Desmarestia (Lamouroux 1813) conducted in the Antarctic ecoregion of Magallanes, it was found this species originated from an Antarctic ancestor, then radiated north, eventually reaching the Northern Hemisphere through long-distance natural dispersal (Peters et al. 1997). Within the sub-Antarctic Chilean ecoregion of Magallanes, the genus Desmarestia is represented by D. confervoides (Bory de Saint-Vincent) M.E. Ramírez & A.F. Peters, D. chordalis J.D. Hooker & Harvey and D. ligulata (Stackhouse) J.V. Lamouroux. Currently, D. distans and D. muelleri are considered subspecies of D. ligulata (Setchell & Gardner 1925; Guiry & Guiry 2014; Yang et al. 2014).
Abbreviations in this article
chl a, b, c: chlorophyll a, b, c
Fucox: fucoxanthin
FW: fresh weight
PAR: photosynthetically active radiation
The representative Desmarestia species in the Antarctic continent are D. anceps Montagne, D. menziesii J. Agardh (Lamb & Zimmermann 1977; Wiencke et al. 1995) and D. antarctica Silva & Moe (Moe & Silva 1989; Wiencke et al. 1991). These Desmarestia species, together with Himantothallus grandifolius (A. Gepp & E.S. Gepp) A.D. Zinova (1959), comprise a large proportion of the macroalgal biomass along the Antarctic Peninsula (Amsler et al. 1995; Brouwer 1996; Quartino et al. 2001; Quartino et al. 2005), yet are largely understudied despite supporting a diverse, associated floral and faunal community.
Photosynthetic pigment composition can reveal potential adaptation and acclimation success, and the physiological traits of macroalgae (Motten 1995; Plastino & Mansilla 2004). A species’ reaction to the given radiation climate is determined by the interaction between genetic adaptation and physiological acclimation where adaptation must precede acclimation to changing environmental conditions (Bischof et al. 2006). That is, the species-dependent and variable genetic preadaptations to limiting, but also potentially harmful, radiation would shape its physiology in the natural environment. For example, in Lessonia nigrescens of the Southern Hemisphere, the photosynthetic responses to seasonally changing irradiance levels are some of its adaptations, along with its ontogenic development (Huovinen et al. 2006). Pigment composition is frequently used as a biomarker to infer physiological adaptations of plants and algae (e.g., Dring 1981; Ramus 1983) to differing environmental variables across bioregions (Falkowski & LaRoche 1991). Laminaria saccharina, for instance, may be capable of optimizing photosynthesis over a wide range of light levels by complex metabolic regulation (Machale et al. 1996). And, under variable irradiance, the red algae Gracilaria lemaneiformis showed differential vegetative and physiological strategies to adapt to environmental stress by regulating different metabolic processes (e.g., photosystem II reaction centres normalization; Wu et al. 2015). The biochemical reactions of photosynthesis are directly coupled to light availability (Plastino & Mansilla 2004), and the respective share of chl a as reaction centre pigment to chl c and other accessory pigments, such as the xanthophyll Fucox, may reveal adaptive responses towards heterogeneous light and climate, fluctuating with latitude, depth, season or a combination of all (Mansilla & Alveal 2004; Plastino & Mansilla 2004). In addition to irradiance and photoperiod (hours daylight: hours darkness), temperature, salinity and nutrients may be further limiting factors at high latitudes, affecting the efficiency of light capture for photosynthesis.
Our objectives were to examine (1) the adjustments or intraspecific differences in pigment composition and ratio (chl c/chl a; chl c/Fucox) in species of the genus Desmarestia spatially from sub-Antarctic to Antarctic marine habitats and (2) the intraspecific differences in pigment concentration in two Antarctic species within the genus Desmarestia (D. antartica, D. menziesii). We hypothesized that primary (chl a) and accessory (chl c and Fucox) pigment composition in Desmarestia species will differ among sites along the latitudinal gradient of sub-Antarctic to Antarctic waters; and along the Antarctic Peninsula, despite site proximity and past connectivity, reflecting response to local conditions such as different duration and intensity of solar irradiance between regions. The overall results of this investigation will contribute to current knowledge and understanding of macroalgal eco-physiology. Marine photosynthetic physiology in sub-Antarctic and Antarctic irradiance regimes is likely to become increasingly relevant in the context of present and future global changes.
Materials and methods
Study sites
Two sites in Chile’s sub-Antarctic region and eight sites along the Antarctic Peninsula were selected to represent a range of environmental conditions (Table 1). We used a Solar Light® radiometer (model: PMA 2200) and a Yellow Spring Instrument® handheld multiparameter (model: MPS) at some sites (e.g., Saint Ana) or a conductivity–temperature–depth recorder (SBE 19plus V2 SEACAT Sea-Bird Electronics Inc.) measurements to first 10 m depth at other sites (e.g., Deception). When not available, approximates of environmental data were obtained from published literature for the given site (e.g., Santana et al. 2006; Clarke et al. 2008; Smith & Comiso 2008; CEAZA-MET 2010; Table 1).
Table 1 Environmental and habitat variables registered in 2011, 2012 and 2013 during collection of five Desmarestia species at 10 study sites in sub-Antarctica and Antarctica. Shown are the mean or mean range values.
| Desmarestia species name |
Study site |
Depth
(m) |
PAR (µE /m2s) |
Salinity
(psu) |
Temperature
(°C) |
Dissolved O2 (mg/L) |
Habitat substrate type |
| Sub-Antarctica |
|
|
|
|
|
|
|
| D. confervoides
|
St Ana Point |
5–7 |
526.4 |
32.53 |
9.73 |
12.8 |
Cliff |
| D. ligulata subsp. ligulata f. distans
|
St Ana Point (S53°08′; W71°00′) |
5–7 |
526.4 |
32.53 |
9.73 |
12.8 |
Cliff |
| D. ligulata subsp. muelleri
|
St Ana Point |
5–7 |
526.4 |
32.53 |
9.73 |
12.8 |
Cliff |
| D. confervoides
|
Paula Bay |
5–7 |
164–165 |
32.17 |
4.7–10.9 |
– |
Boulders |
| D. ligulata subsp. ligulata f. distans
|
Paula Bay (S54°55′; W67°55′) |
5–7 |
164–166 |
32.17 |
4.7–10.9 |
– |
Boulders |
| D. ligulata subsp. muelleri
|
Paula Bay |
5–7 |
164–167 |
32.17 |
4.7–10.9 |
– |
Boulders |
| Antarctica |
|
|
|
|
|
|
|
| D. antarctica
|
Fildes (S62°12′; W58°54′) |
5–7 |
729–787 |
34 |
2 |
10.9 |
Cliff |
| D. menziesii
|
Fildes |
5–7 |
729–787 |
34 |
2 |
10.9 |
Cliff |
| D. anceps
|
Prat (S62°27′; W59°44′) |
9–12 |
748–768 |
33.85 |
2.1 |
8.0 |
Boulders |
| D. menziesii
|
Prat |
5–7 |
748–768 |
33.85 |
2.1 |
8.0 |
Cliff |
| D. antarctica
|
Prat |
5–7 |
748–768 |
33.85 |
2.1 |
8.0 |
Boulders |
| D. anceps
|
Hanna (S62°39′; W60°37′) |
10 |
729–787 |
34 |
1.8 |
7.1 |
Cliff |
| D. antarctica
|
Hanna |
5–7 |
729–787 |
34 |
1.8 |
7.1 |
Cliff |
| D. menziesii
|
Deception (S62°59′; W60°35′) |
5–7 |
901.21 |
33.87 |
2.1 |
8.0 |
Boulders |
| D. antarctica
|
Deception |
5–7 |
901.21 |
33.87 |
2.1 |
8.0 |
Rocky shore |
| D. antarctica
|
Spring (S64°17′; W61°05′) |
5–7 |
729–787 |
33.4 |
0–1 |
– |
Cliff |
| D. menziesii
|
Spring |
5–7 |
729–787 |
33.4 |
0–1 |
– |
Cliff |
| D. menziesii
|
Argentine (S65°14′; W64°15′) |
5–7 |
729–787 |
33.4 |
0–1 |
– |
Cliff |
| D. menziesii
|
Rothera (S67°33′; W68°07′) |
5–7 |
1005.9 |
33.29 |
1.72 |
12.6 |
Cliff |
| D. menziesii
|
Carvajal (S62°12′; W58°54′) |
5–7 |
739.6 |
32.59 |
0.24 |
13.3 |
Cliff |
| D. antarctica
|
Carvajal |
5–7 |
739.6 |
32.59 |
0.24 |
13.3 |
Rocky shore |
Field sample collection
Fronds of Desmarestia confervoides and the subspecies D. ligulata subsp. ligulata f. distans and D. ligulata subsp. muelleri were collected in the austral summer period (December 2011–February 2012) at two sites along the Strait of Magellan to the Beagle Channel (Table 1; Fig. 1). D. antarctica, D. menziesii and D. anceps were collected in Antarctica by scuba diving in February 2013 off Fildes Bay (Fildes), Hanna Point (Hanna), Prat Point (Prat), Deception Island (Deception), Spring Point (Spring), Argentine Islands (Argentine), Rothera Base (Rothera; except D. antarctica, not found) and Carvajal Base (Carvajal; Table 1, Fig. 1). All specimens were placed in freeze–cooler containers to prevent sulfation of Desmarestia individuals and/or degradation of pigments from exposure to light and temperature changes. Sub-Antarctic samples were processed upon collection immediately at the Laboratory of Antarctic and sub-Antarctic Marine Ecosystems, University of Magallanes, in Punta Arenas, Chile. Antarctic samples were transported in coolers, over the short 5- to 10-min Zodiac ride back to our research vessel where they were frozen at −25°C to avoid loss of pigment or similar deterioration for later analysis at the laboratory.
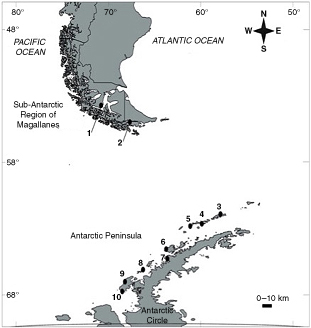
Fig. 1
Study sites sampled for Desmarestia species from which photosynthetic pigments chl a chl c and Fucox were extracted. Shown are the sites in the sub-Antarctic ecoregion: (1) Saint Ana Point (Brunswick Peninsula); (2) Paula Bay (Beagle Channel) and the study sites sampled in the Antarctic Peninsula; (3) Fildes Bay (Fildes); (4) Hanna Point (Hanna); (5) Prat Point (Prat); (6) Deception Island (Deception); (7) Spring Point (Spring); (8) Argentine Island (Argentine); (9) Rothera Base (Rothera); and (10) Carvajal Base (Carvajal).
Pigment analyses
Seven random, individual samples of each species of Desmarestia (7 n×6 species=42) were taken to the laboratory. The frozen samples were thawed, washed with sterilized seawater of salinity 34 psu and were carefully brushed to remove epiphytic organic material. Random ca. 2 cm2 subsamples (0.125 g FW) from the fronds were gently patted with paper towel to remove excess water and weighed in a Radwag analytical balance (AS 220/c/2) for pigment analysis (Seely et al. 1972). Our prior work (unpublished) and that of Seely et al. (1972) showed that dimethyl sulfoxide readily extracts pigments without further addition of acetone. One mL of dimethyl sulfoxide was added to 0.125 g FW (FW and incubated for 15 min in complete darkness). The supernatant was then placed into a 2.5-mL cuvette for spectrophotometric analysis (Genesys 10 UV). No further dilution was necessary. Light absorbance was recorded at wavelengths of 480, 582, 631 and 665 nm once completeness of extraction was determined (white samples).
Pigment concentration was quantified using equations from Seely et al. (1972):


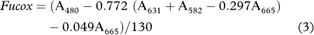
Data analysis
Pigment concentrations were compared among six species (N=7 per species) between sub-Antarctic and Antarctic sites using the Kruskal–Wallis non-parametric test because data were not normally distributed. All conclusions were based on a 95% confidence level (P=0.05). In addition, we calculated pigment ratios for species of the sub-Antarctic region and the Antarctic Peninsula and adjacent islands. All statistical analyses were carried out using the software package STATISTICA, version 7.1 (2004).
Results
Study sites
The sub-Antarctic sites showed lower average salinity and PAR light (32.35 psu and 345.5 µE/m2s, respectively) but generally showed higher average temperature and dissolved oxygen (8.4°C and 12.8 mg/L, respectively) than the sites along the Antarctic Peninsula (33.4 psu, 811.2 µE/m2s, 1.7 °C and 9.6 mg/L; Table 1). Average depth and habitat substrate types where Desmarestia specimen could be collected from for this study remained fairly constant variables across sites in both regions (Table 1).
Spatial and interspecific differences
Overall, higher average concentrations of chl a, chl c and Fucox were observed in Desmarestia species from Antarctic sites compared with sub-Antarctic sites (except D. ligulata subsp. muelleri; Fig. 2a). Among the sub-Antarctic species of Desmarestia, the highest chl a concentration was measured in D. confervoides from Paula Bay (Beagle Channel, Cape Horn Province) and the highest Fucox concentration in D. ligulata subsp. muelleri (lowest chl a) from the Strait of Magellan (Fig. 2a). Chl a concentration differed significantly between D. ligulata subsp. ligulata f. distans and Antarctic D. menziesii (P=0.001; Fig. 2a). The chl c content of sub-Antarctic D. confervoides was significantly different from all Desmarestia species tested (all P <0.002; Fig. 2a). Statistically, Fucox was most variable between the sub-Antarctic species (P=0.009; Fig. 2a).
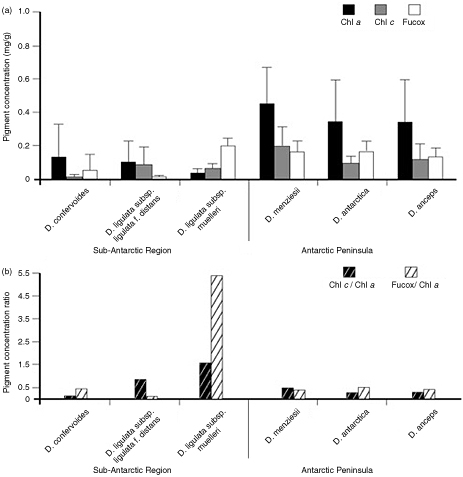
Fig. 2
(a) Mean ± SD pigment concentration (N=7 per species) of chl a, chl c and Fucox and (b) ratios of mean pigment concentration (N=7) of chl c/chl a and of Fucox/chl a in species of the genus Desmarestia from the sub-Antarctic region of Chile and the Chilean Antarctic Peninsula.
The ratio of chl c/chl a was similar in the sub-Antarctic species D. confervoides (1.68), D. ligulata subsp. ligulata f. distans (2.09) and D. ligulata subsp. muelleri (1.61; Fig. 2b). Large differences in the ratio of Fucox/chl a were found between D. ligulata subsp. muelleri, which exhibited an increased proportion of Fucox (5.41 in specimen from Paula Bay, Beagle Channel, Cape Horn; Fig. 2b), and all Desmarestia species tested. The data show similar pigment concentrations and ratios among the Antarctic Desmarestiales and overall fairly lower than in sub-Antarctic species (all between 0.31 and 0.38; Fig. 2b).
Interspecific differences along the Antarctic Peninsula: D. antarctica and D. menziesii.
The higher concentrations of chl a (0.45±0.17 mg.g−1 FW) and chl c (0.20±0.08 mg.g−1 FW) were generally found in D. menziesii. But we measured nearly identical concentrations of Fucox in both, D. menziesii and D. antarctica (Fig. 3a, b).
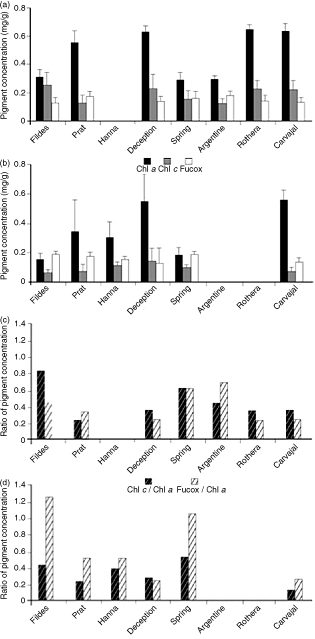
Fig. 3
Pigment concentration of (a) Desmarestia menziesii and (b) D. antarctica along a latitudinal gradient in the Antarctic Peninsula. Shown are the means ± SD (N=7) concentration of chl a, chl c and Fucox, and mean concentration ratios of chl c/chl a and Fucox/chl a in (c) D. menziesii and in (d) D. antarctica from study sites along the Antarctic Peninsula’s latitudinal gradient (N=7).
No clear latitudinal effect was observed for chl a concentration in D. menziesii along the Antarctic Peninsula (Fildes to Carvajal; Table 1, Fig. 3a). High concentration of chl a in D. menziesii was recorded in Deception, followed by Rothera and Carvajal (0.63±0.04, 0.64±0.03 and 0.63±0.05 mg·g−1 FW, respectively), especially at the lowest temperature and salinity sites, near the Antarctic Circle (Table 1, Fig. 3a). A decreasing trend in pigment concentration with increasing latitude was observed from Deception to Spring and Argentine sites. Chl a, chl c and Fucox in D. menziesii at Fildes, Spring and Argentine, with similar abiotic features (Table 1), ranged in similar, and lower, concentrations (all between 0.18 and 0.30±0.03 mg·g−1 FW; Fig. 3a) than sites with variable PAR, temperature, salinity or substrate type (Table 1).
In D. antarctica, chl a concentration varied markedly among sites on the Antarctic Peninsula, the highest chl a concentrations were recorded in Deception (0.56±0.2 mg·g−1 FW) and Carvajal (0.56±0.07 mg·g−1 FW; Fig. 3c, d). Chl c concentration in D. antarctica decreased towards lower latitudes, from the highest values found at Deception (0.15±0.09 mg·g−1 FW) to almost half of the concentration in samples from Fildes Bay. Fucox concentrations barely changed along the latitudinal gradient, between Deception and Spring (Fig. 3b) in spite of different environmental features (Table 1).
We identified overall higher ratios of chl c/chl a in D. menziesii than in D. antarctica, and overall higher ratios of Fucox/chl a in D. antarctica than in D. menziesii considering all locations along the Antarctic Peninsula (Fig. 3c, d). Among samples of D. menziesii, the highest chl c/chl a ratio was registered in Fildes (0.83), the lower latitude site, and the highest Fucox/chl a ratio was registered in Argentine (Fig. 3c). Samples of D. antarctica from locations along the Antarctic latitudinal gradient, showed low chl c/chl a ratio (Fig. 3d). The Fucox/chl a ratios in D. antarctica from Fildes (1.23) and Spring (1.14) were nearly triple (and double in Spring Point) that of D. menziesii (all sites; Fig. 3c, d).
Discussion
We analysed intraspecific and interspecific differences in pigment composition in sub-Antarctic and Antarctic samples of the genus Desmarestia. In the six species studied, we found the lowest concentrations of chl a, chl c and Fucox in the species from sub-Antarctic sites, versus the higher pigment concentrations in species along the Antarctic Peninsula (Table 1; Fig. 3), that is, increasing pigment composition towards the prevailing radiation climate. Though we registered differences between sites, we expected to see a gradient of photosynthetic pigment concentration reflecting the latitudinal gradient along the Antarctic Peninsula. We did not see this pigment gradient from our results (Fig. 3). Such a gradient may have suggested the presence of more accessory pigments in subtidal specimen as a possible strategy to optimize photosynthetic efficiency in regions with shorter light exposure (Gómez et al. 1997). Nevertheless, we saw an important tendency for higher chlorophyll concentrations at our two sites closest to the polar circle (Carvajal and Rothera) and the high-latitude site Deception, especially with D. menziesii (D. antarctica not collected in Rothera). Several biotic and abiotic factors, such as temperature, salinity, nutrients (Ramus 1978; Dhargalkar 2004; Nygård & Dring 2008) and thalli morphology (Peckol & Ramus 1988; Johansson & Snoeijs 2002), may also influence efficiency of light absorption and geographical distribution of macroalgae, survival, growth rates and reproductive success (Van den Hoek 1982; Breeman 1988) at both sub-Antarctic and Antarctic sites.
At lower temperature (usually higher latitude), high rates of photosynthesis are observed among terrestrial and aquatic plants and usually relate to an increased ratio of chl a to b (e.g., Eggert et al. 2006). Similar photosynthetic adaptations are most likely in macroalgae at high latitudes, such as those endemic species of the Antarctic Peninsula. Although the depth of light dispersion influences photosynthetic pigment composition, our samples of Desmarestia species were all collected in the ca. 5 m depth range in sub-Antarctica and Antarctica. It is unlikely that depth was an influential factor on the observed pigment differences. But, the variation in pigment composition among sites on the Antarctic Peninsula may be influenced by the seasonal variation in light, related in turn to variation in temperature, inducing variable pigment production and metabolic rate (e.g., Barko & Smart 1981; Aguilera et al. 2002). Similarly, seasonal sea-ice and snow coverage (Zacher et al. 2009), influencing salinity and nutrients, may also affect the physiology of photosynthesis and algal pigment content (e.g., Nygård & Dring 2008).
Desmarestia’s pigment concentration may vary under different salinities or according to life history stage (Gómez & Wiencke 1996). Salinity changes affect cellular structures responsible for the synthesis and metabolism of proteins, affecting the life cycle and pigment concentration of some brown algae (Nygård & Dring 2008) and are likely to affect Antarctic species of Desmarestia. Nutrients also influence photosynthesis and growth rate. For example, cultivation of Fucus vesiculosus from the Baltic Sea (5 psu) showed higher photosynthetic activity (as electron transfer rate) and relative growth rate at low salinities (<10 psu) than F. vesiculosus from the Irish Sea (35 psu), which decreased sharply in salinities below 20 psu (Nygård & Dring 2008). Fucus specimens from both Baltic (5 psu) and Irish (20 psu) sites under high-nutrient conditions performed better at low salinities than those grown in low-nutrient cultures (Nygård & Dring 2008). Salinity may therefore be a stronger influence on differences in photosynthesis (and growth) between specimens from two geographic regions or populations, followed by differences in nutrient concentrations. A marked coastal isocline is more characteristic of Antarctic than of sub-Antarctic waters (Palma et al. 2008) and this might explain the overall lower pigment concentration and ratios in Desmarestia from sub-Antarctic sites than in those from Antarctica. An increase in global temperatures, with its consequent increase in glacial inflows, reduced salinity and increased water turbidity, may alter depth distribution patterns—and therefore productivity—of macroalgal communities, especially in high-latitude regions.
Though salinity barely differed between sites in both study regions, macroalgal pigment composition and concentration may also vary according to life history stage and thallus morphology (Gómez et al. 1997; Wiencke, Clayton et al. 2006). Early stages of D. menziesii would be better adapted to low light intensities than adult stages because they have more pigment composition per unit of biomass and therefore more investment in light-harvesting mechanisms (Gómez 2001). Alternatively, the Desmarestia species we examined have filamentous and laminar thalli, which directly affect the efficiency of light absorption (Ramus 1978; Peckol & Ramus 1988; Johansson & Snoeijs 2002). Thinner species have increased photosynthetic tissue per unit biomass. In D. menziesii, the content of chl a, c and Fucox in cultured samples increased with thallus biomass (Gómez & Wiencke 1997). The pigment composition of D. confervoides, of cylindrical and branched thalli morphology, showed a photosynthetic capacity between that of fleshy and laminar thalli with few cell layers (Rautenberger et al. 2009). This morphology is typical of sub-Antarctic species. Therefore, the greatest concentration of Fucox in D. ligulata subsp. muelleri (and D. antarctica, similar morphology) may be due to the laminar thallus morphology potentially influencing pigment concentrations (Eggert et al. 2006; Navarro et al. 2009) in these sub-Antarctic algae.
In the sub-Antarctic species, no clear pattern in pigment composition was observed from Saint Ana Point to Paula Bay near Cape Horn (Drake Passage; Fig. 1). The sub-Antarctic marine habitats are exposed to the unique confluence of three ocean influences (Reid 1989) where heavy precipitation, for instance, may set a subtle but important difference among study regions (Table 1), in addition to fluvial run off often rare in sites of the Antarctic Peninsula. This may explain the lower water salinity and higher temperatures registered in general in the physico-chemically heterogeneous sub-Antarctic environment. The greater variation in, and generally lower, pigment concentration among the sub-Antarctic Desmarestiales studied may result from low ice or cloud cover, decreased water turbidity from glacial run off, longer daylength in winter or large heterogeneity in the physico-chemical environment (e.g., the advance and retreat of ice [Silva & Calvete 2002]).
We found differences in pigment concentration, especially in chl a, between our study sites along the Antarctic Peninsula (Fildes, Prat, Deception, Spring, Argentine, Rothera and Carvajal), although the concentrations of Fucox barely changed among sites. This might be explained by the fact that accessory pigments (carotenoids) are generally less sensitive to severe environments compared with chl a (Häder & Häder 1989). Antarctic macroalgae may be better acclimated than other algae to withstand environmental extremes (irradiance, photoperiod, temperature, salinity, nutrients) that affect the photosynthetic efficiency and pigment composition in high latitudes (Gómez et al. 1997; Gómez 2001; Wiencke, Roleda et al. 2006).
Though we did not measure directly all possible abiotic conditions (cloud cover, transient ice), in other studies, reduced availability of PAR due to increased water turbidity from glacial influence and depth in coastal Antarctica led to decreased macroalgal vertical distribution, photosynthetic parameters (Rautenberger et al. 2013) and metabolic carbon balance (Deregibus et al. 2015). Similar works reveal that the duration of daily exposure, rather than the intensity of light, is most significant for macroalgal productivity and photosynthetic efficiency in coastal areas (Gómez et al. 1997; Zacher et al. 2009). That is, the increased pigment concentration in Antarctic algae during the summer may be a strategy to increase light-harvesting efficiency in an environment with significantly less annual solar radiation than that in temperate to tropical regions (Ramus 1981; Kirst & Wiencke 1995; Wiencke, Roleda et al. 2006).
In contrast, lower pigment content could protect individuals in regions (e.g., sub-Antarctic) with less-intense light regime (though available over longer time, that is, months per year, daylength in winter) than the short-and-intense Antarctic light regime in the summer (e.g., Gómez et al. 1997; Gómez 2001). Similar patterns have been found in a number of seaweeds from a high Arctic site (Aguilera et al. 2002). Samples were only collected in summer and therefore we were unable to test this potential strategy. In addition, photoperiod is suggested to influence the concentration of accessory pigments in Antarctic waters to a greater degree than it influences chl a (Yokoya et al. 2007). But, under field conditions, the effects of photoperiod and irradiance are difficult to disentangle, and thus further studies under controlled laboratory conditions are recommended. It is likely that changes occur in pigment composition of the photosynthetic antenna in sub-Antarctic Desmarestia (e.g., decrease in chl a with increased light availability in summer; our unpubl. data), but its physiological response must be further studied.
In conclusion, despite the environmental extremes of polar waters, macroalgae show physiological adaptations (Wiencke 1990). These adaptations can be seen in the photosynthetic apparatus and pigment composition (Kirst & Wiencke 1995). Amid the cumulative global and environmental changes, including increases in average temperatures—and consequent changes in salinity, light intensity and availability—there is a growing need for basic research to guide applied studies (e.g., biotechnology) using algae and their mechanisms of adaptation, survival and irradiance protection as model machinery.
Acknowledgements
This research was funded by Chile’s National Council for Research in Science and Technology (CONICYT) Program FONDECYT, project 1140940, the Millennium Scientific Initiative (grant P05-002 ICM, Chile) and the Basal Financing Program of the (grant PFB-23, Chile). We thank the resources and support offered by the Chilean Navy Ships AP-46 ″Almirante Óscar Viel, and AP–41 Aquiles during field expeditions. We are thankful for the graduate scholarships by the Institute of Ecology and Biodiversity granted to FM (ICM, P05-002) and JPR (ICM, P05-002) and to JM (PFB-23-2008) and SR (ICM P05-002).
References
Aguilera
J.,
Bischof
K.,
Karsten
U.,
Hanelt
D.
&
Wiencke
C.
2002.
Seasonal variation in ecophysiological patterns in macroalgae from an Arctic fjord. II. Pigment accumulation and biochemical defence systems against high light stress. Marine Biology 140, 1087–1095.
Publisher Full Text
Amsler
C.,
Rowley
R.,
Laur
D.,
Quetin
L.
&
Ross
R.
1995.
Vertical distribution of Antarctic peninsular macroalgae: cover, biomass and species composition. Phycologia 34, 424–430.
Publisher Full Text
Aravena
J.
&
Luckman
B.
2009.
Spatio-temporal rainfall patterns in southern South America. International Journal of Climatology 29, 2106–2120.
Publisher Full Text
Barko
J.W.
&
Smart
R.M.
1981.
Comparative influences of light and temperature on the growth and metabolism of selected submersed freshwater macrophytes. Ecological Monographs 51, 219–236.
Publisher Full Text
Bischof
K.,
Rautenberger
R.,
Brey
L.
&
Pérez-Lloréns
J.L.
2006.
Physiological acclimation to gradients of solar irradiance within mats of the filamentous green macroalga Chaetomorpha linum from southern Spain. Marine Ecology Progress Series 306, 165–175.
Publisher Full Text
Breeman
A.
1988.
Relative importance of temperatures and other factors in determining geographic boundaries of seaweeds: experimental and phenological evidence. Helgoländer Meeresunters 42, 199–241.
Publisher Full Text
Brouwer
P.
1996.
Decomposition in situ of the sublittoral Antarctic macroalga Desmarestia anceps Montagne. Polar Biology 16, 129–137.
Publisher Full Text
CEAZA-MET (Center for Advanced Studies in Arid Zones, Meteorology Section) 2010. Red Meteorológica de Sensores, Chile. (Network of Meteo–Sensors, Chile). Accessed on the internet at http://www.ceazamet.cl on February 2013.
Clarke
A.,
Meredith
M.P.,
Wallace
M.I.,
Brandon
M.A.
&
Thomas
D.N.
2008.
Seasonal and interannual variability in temperature, chlorophyll and macronutrients in northern Marguerite Bay, Antarctica. Deep Sea Research Part II 55, 1988–2006.
Publisher Full Text
Dayton
P.
1985.
Ecology of kelp communities. Annual Review of Ecology and Systematics 16, 215–245.
Publisher Full Text
Deregibus
D.,
Quartino
M.L.,
Campana
G.L.,
Momo
F.R.,
Wiencke
C.
&
Zacher
K.
2015.
Photosynthetic light requirements and vertical distribution of macroalgae in newly ice-free areas in Potter Cove, South Shetland Islands, Antarctica. Polar Biology 39, 153–166.
Publisher Full Text
Dhargalkar
V.
2004.
Effect of different temperature regimes on the chlorophyll a concentration in four species of Antarctic macroalgae. Seaweed Research and Utilization 26, 237–243.
Dring
M.J.
1981.
Chromatic adaptation of photosynthesis in benthic marine algae: an examination of its ecological significance using a theoretical model. Limnology and Oceanography 26, 271–284.
Publisher Full Text
Eggert
A.,
Visser
R.J.W.,
Van Hasselt
P.R.
&
Breeman
A.M.
2006.
Differences in acclimation potential of photosynthesis in seven isolates of the tropical to warm temperate macrophyte Valonia utricularis (Chlorophyta). Phycologia 45, 546–556.
Publisher Full Text
Falkowski
P.G.
&
LaRoche
J.
1991.
Acclimation to spectral irradiance in algae. Journal of Phycology 27, 8–14.
Publisher Full Text
Gómez
I.
2001.
Ecophysiology of Antarctic macroalgae: effects of environmental light conditions on photosynthetic metabolism. Revista Chilena Historia Natural 74, 251–271.
Gómez
I.
&
Wiencke
C.
1996.
Photosynthesis, dark respiration and pigment contents of gametophytes and sporophytes of the Antarctic brown alga Desmarestia menziesii. Botanica Marina 39, 149–158.
Gómez
I.
&
Wiencke
C.
1997.
Seasonal growth and photosynthetic performance of the Antarctic macroalga Desmarestia menziesii (Phaeophyceae) cultivated under fluctuating Antarctic daylengths. Botanica Acta 110, 25–31.
Publisher Full Text
Gómez
I.,
Weykam
G.,
Klöser
H.
&
Wiencke
C.
1997.
Photosynthetic light requirements, metabolic carbon balance and zonation of sublittoral macroalgae from King George Island (Antarctica). Marine Ecology Progress Series 148, 281–293.
Publisher Full Text
Guiry
M.
&
Guiry
G. 2014. AlgaeBase. Accessed on the internet at http://www.algaebase.org on 22 December 2014.
Häder
D.P.
&
Häder
M.
1989.
Effects of solar UV-B irradiation on photomovement and motility in photosynthetic and colourless flagellates. Environmental and Experimental Botany 29, 273–282.
Publisher Full Text
Huovinen
P.,
Gómez
I.
&
Lovengreen
C.
2006.
A five-year study of solar ultraviolet radiation in southern Chile (39° S): potential impact on physiology of coastal marine algae? Photochemistry and Photobiology 82, 515–522.
PubMed Abstract | Publisher Full Text
Johansson
G.
&
Snoeijs
P.
2002.
Macroalgal photosynthetic responses to light in relation to thallus morphology and depth zonation. Marine Ecology Progress Series 244, 63–72.
Publisher Full Text
Kirst
G.
&
Wiencke
C.
1995.
Ecophysiology of polar algae. Journal of Phycology 31, 181–199.
Publisher Full Text
Lamb
I.
&
Zimmerman
M.H. 1977. Benthic marine algae of the Antarctic Peninsula.
Washington,
DC: American Geophysical Union.
Lamouroux
J.V.F. 1813. Essai sur les genres de la famille des thalassiophytes non articulées. (Essay on the genera of the non-articulated thalassiophytes family.) Annales du Muséum d’Histoire Naturelle, Paris 20, 21–47, 115–139, 267–293, pls 7–13.
Machale
K.M.,
Davison
I.R.
&
Falkowski
P.G.
1996.
Thermal acclimation and photoacclimation of photosynthesis in the brown alga Laminaria saccharina. Plant, Cell & Environment 19, 1005–1016.
Publisher Full Text
Mansilla
A.
&
Alveal
K.
2004.
Generalidades sobre las macroalgas. (Overview of macroalgae.)
In C. Werlinger (ed.): Biología marina y oceanografía: conceptos y procesos. (Marine biology and oceanography: concepts and processes.) Pp. 349–362. Concepción: Trama Impresores S.A.
Moe
R.L.
&
Silva
P.C.
1989. Desmarestia antarctica (Desmarestiales, Phaeophyceae), a new ligulate Antarctic species with an endophytic gametophyte. Plant Systematics and Evolution 164, 273–283.
Publisher Full Text
Motten
A.F.
1995.
Diversity of photosynthetic pigments.
In C.A. Goldman (ed.): Tested studies for laboratory teaching. Proceedings of the workshop/conference of the Association for Biology Laboratory Education (ABLE). Vol. 16. Pp. 81–98. New Haven, CT: Association for Biology Laboratory Education.
Navarro
N.P.,
Mansilla
A.
&
Plastino
E.
2009. Iridaea cordata (Gigartinales, Rhodophyta): responses to artificial UVB radiation. Journal of Applied Phycology 22, 385–394.
Publisher Full Text
Nygård
C.
&
Dring
M.J.
2008.
Influence of salinity, temperature, dissolved inorganic carbon and nutrient concentration on the photosynthesis and growth of Fucus vesiculosus from the Baltic and Irish Seas. European Journal of Phycology 43, 253–262.
Publisher Full Text
Palma
E.D.,
Matano
R.P.
&
Piola
A.R. 2008. A numerical study of the southwestern Atlantic shelf circulation: stratified ocean response to local and offshore forcing. Journal of Geophysical Research—Oceans
113, C11010, doi: 10.1029/2007JC004720.
Publisher Full Text
Peckol
P.
&
Ramus
J.
1988.
Abundances and physiological properties of deep-water seaweeds from Carolina outer continental shelf. Journal of Experimental Marine Biology and Ecology 115, 25–39.
Publisher Full Text
Peters
A.F.,
Oppen
M.J.,
Wiencke
C.,
Stam
W.T.
&
Olsen
J.L.
1997.
Phylogeny and historical ecology of the Desmarestiaceae (Phaeophyceae) support a Southern Hemisphere origin. Journal of Phycology 33, 294–309.
Publisher Full Text
Plastino
E.
&
Mansilla
A.
2004.
Luz y fotosíntesis. (Light and photosynthesis.)
In C. Werlinger (ed.): Biología marina y oceanografía: conceptos y procesos. (Marine biology and oceanography: concepts and processes.) Pp. 229–252. Concepción: Trama Impresores S.A.
Quartino
M.,
Kloeser
H.,
Schloss
I.
&
Wiencke
C.
2001.
Biomass and associations of benthic marine macroalgae from the inner Potter Cove (King George Island, Antarctica) related to depth and substrate. Polar Biology 24, 349–355.
Publisher Full Text
Quartino
M.,
Zaixso
H.E.
&
Boraso de Zaixso
A.L.
2005.
Biological and environmental characterization of marine macroalgal assemblages in Potter Cove, South Shetland Islands, Antarctica. Botanica Marina 48, 187–197.
Publisher Full Text
Ramus
J.
1978.
Seaweed anatomy and photosynthetic performance: the ecological significance of light guides, heterogenous absorption and multiple scatter. Journal of Phycology 14, 352–362.
Publisher Full Text
Ramus
J. 1981. The capture and transduction of light energy. In
C.S.
Lobban,
M.J.
Wynne (eds.): The biology of seaweeds. Pp. 458–492.
Berkely,
CA: University of California Press.
Ramus
J.
1983.
A physiological test of the theory of complementary chromatic adaptation. II. Brown, green and red seaweeds. Journal of Phycology 19, 173–178.
Publisher Full Text
Rautenberger
R.,
Mansilla
A.,
Gómez
I.,
Wiencke
C.
&
Bischof
K.
2009.
Photosynthetic responses to UV-radiation of intertidal macroalgae from the Strait of Magellan (Chile). Revista Chilena de Historia Natural 82, 43–61.
Publisher Full Text
Rautenberger
R.,
Wiencke
C.
&
Bischof
K.
2013.
Acclimation to UV radiation and antioxidative defence in the endemic Antarctic brown macroalga Desmarestia anceps along a depth gradient. Polar Biology 36, 1779–1789.
Publisher Full Text
Reid
J.L.
1989.
On the total geostrophic circulation of the South Atlantic Ocean: flow patterns, tracers, and transports. Progress in Oceanography 23, 149–244.
Publisher Full Text
Santana
A.,
Porter
C.,
Butorovic
N.
&
Olave
C.
2006.
Primeros antecedentes climatológicos de estaciones automáticas (AWS) en el canal Beagle, Magallanes, Chile. (First climatic registry by automatic weather stations [AWS] in Beagle Channel, Magallanes, Chile.) Anales del Instituto de la Patagonia 34, 5–20.
Seely
G.R.,
Duncan
M.J.
&
Vidiver
W.E.
1972.
Preparative and analytical extraction of pigments from brown algae with dimethyl sulfoxide. Marine Biology 12, 184–188.
Publisher Full Text
Setchell
W.A.
&
Gardner
N.L.
1925.
The marine algae of the Pacific coast of North America. University of California Publications in Botany 8, 383–898.
Silva
N.
&
Calvete
C.
2002.
Características oceanográficas físicas y químicas de canales australes chilenos entre el golfo de Penas y el estrecho de Magallanes. CIMAR Dos Fiordos. (Physical and chemical oceanographic characteristics of southern Chilean channels between the Gulf of Penas and the Strait of Magellan. CIMAR Two Fjords.) Ciencia y Tecnologia del Mar 25, 23–88.
Smith
W.O.
Jr.
&
Comiso
J.C.
2008.
The influence of sea ice on primary production in the Southern Ocean: a satellite perspective. Journal of Geophysical Research 113, C05S93.
StatSoft. Inc. 2004. STATISTICA (data analysis software system) version 7.1. Accessed on the internet at www.statsoft.com/Products/STATISTICA/ in June–November 2014.
Torres
R.,
Silva
N.,
Reid
B.
&
Frangopulos
M.
2014.
Silicic acid enrichment of Subantarctic surface water from continental inputs along the Patagonian Archipelago interior sea (41–56 S). Progress in Oceanography 129, 50–61.
Publisher Full Text
Valdenegro
A.
&
Silva
N.
2003.
Caracterización oceanográfica física y química de la zona de canales y fiordos australes de chile entre el Estrecho de Magellan y Cabo de Hornos. CIMAR Tres Fiordos. (Oceanographic physical and chemical characterization of the area of channels and fjords of Chile between the Strait of Magellan and Cape Horn. CIMAR Three Fjords.) Ciencia y Tecnología Marina 26, 19–60.
Van den Hoek
V.D.C.
1982.
The distribution of benthic marine algae in relation to the temperature regulation of their life histories. Biological Journal of the Linnean Society 18, 81–144.
Publisher Full Text
Wiencke
C.
1990.
Seasonality of brown macroalgae from Antarctica—a long-term culture study under fluctuating Antarctic daylengths. Polar Biology 10, 589–600.
Publisher Full Text
Wiencke
C.,
Clayton
M.N.,
Gómez
I.,
Iken
K.,
Lüder
U.H.,
Amsler
C.D.,
Karsten
U.,
Hanelt
D.,
Bischof
K.
&
Dunton
K.
2006.
Life strategy, ecophysiology and ecology of seaweeds in polar waters. Reviews in Environmental Science and Biotechnology 6, 95–126.
Publisher Full Text
Wiencke
C.,
Clayton
M.N.
&
Schulz
D.
1995.
Life history, reproductive morphology and development of the Antarctic brown alga Desmarestia menziesii J. Agardh. Botanica Acta 108, 201–208.
Publisher Full Text
Wiencke
C.,
Roleda
M.Y.,
Gruber
A.,
Clayton
M.N.
&
Bischof
K.
2006.
Susceptibility of zoospores to UV radiation determines upper depth distribution limit of Arctic kelps: evidence through field experiments. Journal of Ecology 94, 455–463.
Publisher Full Text
Wiencke
C.,
Stolpe
U.
&
Lehmann
H.
1991.
Morphogenesis of the brown alga Desmarestia antarctica cultivated under seasonally fluctuating Antarctic daylengths. Serie Scientifica Chilean Antarctic Institute (INACH) 41, 65–78.
Wu
H.,
Jiang
H.,
Liu
C.
&
Deng
Y.
2015.
Growth, pigment composition, chlorophyll fluorescence and antioxidant defenses in the red alga Gracilaria lemaneiformis (Gracilariales, Rhodophyta) under light stress. South African Journal of Botany 100, 27–32.
Publisher Full Text
Yang
E.C.,
Peters
A.F.,
Kawai
H.,
Stern
R.,
Hanyuda
T.,
Bárbara
I.,
Müller
D.G.,
Strittmatter
M.,
Prud’homme van Reine
W.F.
&
Küpper
F.C.
2014.
Ligulate Desmarestia (Desmarestiales, Phaeophyceae) revisited: D. japonica sp. nov. and D. dudresnayi differ from D. ligulata. Journal of Phycology 50, 149–166.
PubMed Abstract | Publisher Full Text
Yokoya
N.S.,
Necchi
O.,
Martins
A.P.,
Gonzalez
S.F.
&
Plastino
E.M.
2007.
Growth responses and photosynthetic characteristics of wild and phycoerythrin-deficient strains of Hypnea musciformis (Rhodophyta). Journal of Applied Phycology 19, 197–205.
Publisher Full Text
Zacher
K.,
Roleda
M.Y.,
Wulff
A.,
Hanelt
D.
&
Wiencke
C.
2009.
Responses of Antarctic Iridaea cordata (Rhodophyta) tetraspores exposed to ultraviolet radiation. Psychological Research 57, 186–193.
Zinova
A.D.
1959.
O dvukh burykh vodoroslyakh iz Antarktiki: Phyllogigas i Himantothallus. (On two brown algae from Antarctica: Phyllogigas and Himantothallus.) Botanicheski Zhurnal (Botanical Journal) 44, 372–379.